Evidence that stratospheric ozone is destroyed by chlorine and bromine
Numerous laboratory investigations and analyses of worldwide measurements made in the stratosphere have demonstrated that chlorine- and bromine-containing chemicals destroy ozone molecules.
Research studies in the laboratory show that chlorine (Cl) reacts very rapidly with ozone. They also show that the reactive chemical chlorine monoxide (ClO) formed in that reaction can undergo further processes that regenerate the original chlorine, allowing the sequence to be repeated very many times (a chain reaction). Similar reactions also take place between bromine and ozone.
But do these ozone-destroying reactions occur in the "real world"? All the accumulated scientific experience demonstrates that the same chemical reactions do take place in nature. Many other reactions (including those of other chemical species) are often also taking place simultaneously in the stratosphere. This makes the connections among the changes difficult to untangle. Nevertheless, whenever chlorine (or bromine) and ozone are found together in the stratosphere, the ozone-destroying reactions are taking place.
Sometimes a small number of chemical reactions are so dominant in the natural circumstance that the connections are almost as clear as in laboratory experiments. Such a situation occurs in the Antarctic stratosphere during the springtime formation of the ozone hole. Independent measurements made by instruments from the ground and from balloons, aircraft, and satellites have provided a detailed understanding of the chemical reactions in the Antarctic stratosphere. Large areas reach temperatures so low (less than 80oC, or 112oF) that stratospheric clouds form, which is a rare occurrence, except during the polar winters. These polar stratospheric clouds allow chemical reactions that transform chlorine species from forms that do not cause ozone depletion into forms that do cause ozone depletion. Among the latter is chlorine monoxide, which initiates ozone destruction in the presence of sunlight. The amount of reactive chlorine in such regions is therefore much higher than that observed in the middle latitudes, which leads to much faster chemical ozone destruction. The chemical reactions occurring in the presence of these clouds are now well understood from studies under laboratory conditions that mimic those found naturally in the atmosphere.
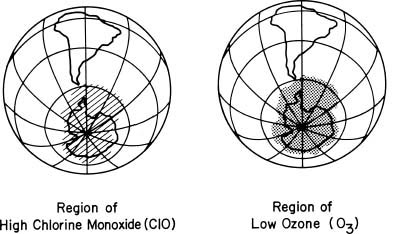
Scientists have repeatedly observed a large number of chemical species over Antarctica since 1986. Among the chemicals measured were ozone and chlorine monoxide, which is the reactive chemical identified in the laboratory as one of the participants in the ozone-destroying chain reactions. The satellite maps shown in the figure below relate the accumulation of chlorine monoxide observed over Antarctica and the subsequent ozone depletion that occurs rapidly in a few days over very similar areas.
Similar reactions involving chlorine and bromine have also been shown to occur during winter and spring in the Arctic polar regions, which leads to some chemical depletion of ozone in that region. Because the Arctic is not usually as persistently cold as the Antarctic, fewer stratospheric clouds form, and therefore there is less ozone depletion in the Arctic, which is the subject of a later question.
Chlorine Monoxide and the Antarctic Ozone Hole: Late August 1996