A
Review of Arsenic in Ambient Air in the UK
A
report produced for the Department of the Environment, Transport and the
Regions, the Scottish Executive and The National Assembly for Wales
Prepared By Richard Maggs - Principal Consultant, Stanger Science and Environment
February
2000
Download
a copy of the report in Word 97 format here (including figures)
Executive Summary
This report
reviews the occurrence of arsenic (As) as an atmospheric pollutant
in the UK. Specifically, it considers the sources of emissions of arsenic
in the UK and reviews the available data on ambient arsenic concentrations
in the UK atmosphere. Consideration is given to the measurement methods
for the determination of arsenic concentrations in suspended particulate
matter; the most appropriate capturing medium for particulate capture;
and the subsequent analytical methods employed for the determination of
arsenic concentrations. The occurrence of vapour phase arsenic is also
discussed, alongside recent developments in analytical procedures for the
measurement thereof.
The review
highlights that ambient concentrations of particulate-bound arsenic have
been declining over the last two decades. This downward trend reflects
the decrease in emissions of arsenic in the UK, notably as a consequence
of changes in the power generation sector where switches have been made
from traditional coal-burning facilities to those that now burn natural
gas as a cheaper alternative. In addition, advances in clean technology
and a greater regulation of industrial processes, both through Integrated
Pollution Control (IPC) and Local Authority Air Pollution Control (LAAPC),
have facilitated this decline.
Currently,
annual mean concentrations of arsenic in the UK rural environment are typically
in the range of 1 - 4 ng m-3, whilst annual mean urban concentrations
are higher, in the range of 5 - 7 ng m-3. Typically, the highest
concentrations of arsenic in the UK are found at sites located in the immediate
vicinity of industrial processes such as smelters, incinerators and cement
works. For example, the highest reported concentrations of arsenic in the
UK have been found in the vicinity of a smelter in Walsall, West Midlands.
For the monitoring period covering 1975 - 1990, the highest annual mean
concentration of arsenic reported at the site was 223 ng m-3,
whilst for other urban sites, during the same period, annual mean values
were found to vary from 1 - 18 ng m-3. The highest reported
concentration (quarterly mean) of arsenic found at the Walsall site was
573 ng m-3.
Current gaps
in the knowledge of arsenic occurrence have been identified in the current
work. Notably, there is a lack of information surrounding monitored levels
of arsenic in Scotland and Wales – concentrations reported here concern
mostly the Midlands area in England. Moreover, there is a lack of information
surrounding the identification of different arsenic species in air - to
date, only total arsenic concentrations have been reported - and the reporting
of vapour-phase concentrations (as arsenic trioxide (AsO3).
In the case of the latter it is envisaged that this is only a problem in
respect of occupational exposure or in the vicinity of industrial processes
where methylated forms (monomethylarsonic acid (MMA) and dimethylarsinic
acid (DMA)) of arsenic may occur. For population exposure, it has been
shown that it is the ultrafine fraction of particulates (PM2.5)
for which arsenic compounds are mostly bound.
The review
highlights that there is currently a paucity in monitoring of arsenic in
the UK. Specifically, the inclusion of various industrial sectors that
may be emitting relatively high levels of arsenic at a local scale. A recent
programme of monitoring has begun at 30 sites within the vicinity of various
industrial processes to determine ambient concentrations of lead and heavy
metals (of which arsenic is included) in the UK. The programme, funded
by the Department of the Environment, Transport and the Regions and carried
out by consultants, Stanger Science and Environment, has been initiated
in response to the recent Review of the UK National Air Quality Strategy
(NAQS), and in anticipation of the future EC Daughter Directive which will
aim to set limit values for a number of pollutants, including heavy metals.
The programme is scheduled to run for a period of 12 months. It is anticipated
that a full set of results will be made available in December 2000.
1
Introduction
This report
has been prepared to assess the current knowledge of arsenic as an atmospheric
pollutant in the UK. The atmospheric chemistry and sources of arsenic emissions
are considered, alongside current guidelines on acceptable limits for the
protection of human health. Measurement techniques and the results of previous
monitoring programmes for arsenic carried out in the UK are also discussed.
A
technical annex is included in this review which gives a detailed overview
of the properties of arsenic and its various compounds and the various
issues relating to its presence in air. These issues are dealt with only
broadly in the following section.
2
Physical and Chemical Nature of Arsenic
2.1
Properties of Arsenic
Elemental arsenic
(As) is a silver-grey crystalline metallic solid that exhibits low
thermal conductivity. Although arsenic is often referred to as a metal,
it is classified chemically as a non-metal or metalloid belonging to Group
15 (VA) of the periodic table. The principle valances of arsenic are +3,
+5 and -3. The main physical properties of arsenic are presented in Table
2.1 below.
Table 2.1:
Physical Properties of Arsenic
| Property |
Value |
| Atomic
weight |
74.92 |
| Melting
point |
816
oC |
| Boiling
point |
615
oC |
| Specific
gravity (26° C) |
5,778
kg/m3 |
| Specific
heat |
24.6
J/(mol.K) |
| Latent
heat of fusion |
27,740
cal/g |
| Latent
heat of sublimation |
31,974
cal/mol |
| Linear
coefficient of thermal expansion (20° C) |
5.6
m m/m° C |
| Electrical
resistivity (0° C) |
26
mW /cm |
| Crystal
system |
hexagonal
(rhombohedral) |
| Lattice
constants (26° C, mm) |
a
= 0.376 e = 1.0548 |
‘Metallic’
arsenic remains stable in dry air, but its surface will oxidise when exposed
to humid air, creating a superficial bronze tarnish that turns black upon
prolonged exposure. It typically exists in two forms; the ‘alpha’ crystalline
metallic form which is steel grey in appearance and brittle in nature,
and the ‘beta’ form; a dark grey amorphous solid.
2.2
Occurrence of Arsenic
Arsenic is
found widely in nature, most often combined with oxygen, chlorine and sulphur.
It is found in trace quantities in all living things, the atmosphere, water
and geological formations. It is usually found in ores containing gold,
silver, cobalt, nickel and antimony. There are over 150 known arsenic-bearing
minerals, the most common of which are summarised in Table 2.2 below.
Table
2.2 : Important Arsenic-Bearing Minerals
| Mineral |
Arsenic
Content,
% |
| Arsenopyrite |
46 |
| Lollingite |
73 |
| Orpiment |
61 |
| Realger |
70 |
| Native
Arsenic |
90
- 100 |
Compounds
of arsenic can be typically divided into two categories : inorganic and
organic forms. Inorganic arsenic occurs naturally in many kinds of rocks
as highlighted in Table 2.2 - the most commonly found inorganic form is
with sulphide ores, such as arsenopyrite.
Organic compounds
of arsenic occur due to its affinity as an element to combine easily with
carbon to form a wide variety of organic compounds with one or more As-C
bonds. Organic species of arsenic in air are considered to be negligible,
although the most commonly occurring organic forms are monomethylarsonic
acid (MMA) and dimethylarsinic acid (DMA).
The main inorganic
and organic compounds of arsenic are dealt with in more detail in Annex
2.
3
The Atmospheric Chemistry of Arsenic
Arsenic is
released to air from natural sources (e.g., volcanoes and forest fires)
and from anthropogenic emissions from various industrial sources (e.g.
coal combustion, smelter and mining activities) and pesticide application.
Annual global emissions from natural sources have been estimated to be
approximately 8,000 tonnes per year (Becher and Wahrendorf, 1992; WHO,
1987) whilst emissions from anthropogenic sources have been estimated to
be approximately three times higher, in the region of 23,600 tonnes per
year (WHO, 1987). It is estimated that a total of 575 tonnes of arsenic
are emitted to the atmosphere in Europe (1990) most of which occur as a
result of the combustion of fossil fuels from stationary sources.
In the United
Kingdom, estimated total annual arsenic emissions in 1996 were 51 tonnes
(NAEI, 1997), although these have been decreasing steadily to levels of
around 8 tonnes per annum in 1998 (Pollution Inventory, 1998) as a result
of an increase in the use of natural gas for power generation in favour
of coal burning.
The use of
organic arsenic compounds in pesticides and herbicides used to be an important
source of arsenic emissions to the atmosphere. However, as arsenic-based
pesticides, specifically insecticides, have been gradually replaced by
alternatives, emissions have significantly decreased in certain parts of
the world (WHO (in preparation); Becher and Wahrendorf, 1992).
3.1 Particulate Arsenic
Arsenic in
air primarily exists in the form of various compounds adsorbed onto the
surface of fine particles (mostly in particles less than 2 m m in diameter)
and is usually a mix of arsenite and arsenate (see Annex
2).
These particles
can be transported by wind and air currents until they are brought back
to earth by wet or dry deposition. The residence time of particulates depends
not only on the size of the particles and the prevailing meteorological
conditions, but also the operational conditions of the industrial process.
For example, levels of arsenic in air vary according to the distance from
the source; the height of the stack; the exit velocity of the flue gas
and the prevailing wind speed to name just a few parameters. Areas in close
proximity to non-ferrous metal smelters have reported high concentrations
of arsenic in air (Lee et al., 1994).
In general,
larger urban conurbations have higher arsenic-in-air concentrations than
smaller ones. Historically, this has been due to the contribution of emissions
from domestic coal burning although recent shifts in the number of households
burning coal in the UK mean that this is no longer necessarily the case.
Due to the
accumulative nature of arsenic, the long-term effects rather than the short-term
effects are those that are of most concern in the UK and other countries.
Deposition studies facilitate the understanding of accumulation and likely
occurrence of damaging effects of metals and metalloid species in the environment.
The effective dry deposition rate of the average arsenic-containing aerosol
is about 0.2 cm s-1 in the vicinity of emission sources. During
transport in the atmosphere, the deposition rate decreases due to preferential
removal of larger particles. A representative deposition rate of 0.1 cm
s-1 is found for arsenic in outdoor air (Slooff et al, 1990).
For the Netherlands, the removal through dry deposition takes place at
an average rate of 0.5% per hour and through wet deposition at 1.2-1.5%
per hour. From this, a mean lifetime of atmospheric arsenic aerosol of
about 2.5 days can be calculated, allowing for arsenic aerosol to be transported
over distances of 1000 km or more (Slooff et al.,1990).
For the Netherlands,
during the period 1978-1982, dry deposition has been estimated at 70-220
m g m-2 yr-1 and wet deposition i.e., the wash-out
of from the atmosphere in rainwater, has been estimated at 0.75-1.5 m g
l-1 yr-1 - equivalent to about 400-680 m g m-2
yr-1 (Slooff et al.,1990). Elsewhere in Europe, deposition values
have been determined for a number of discreet areas. For example, in Germany,
the dry deposition rates for arsenic vary from about 0.5 m g m-2
day-1 in remote areas; below 3 m g m-2 day-1
in rural areas; 3-9 m g m-2 day-1 in urban areas,
and >15 m-2 day-1 in the vicinity of emission sources
(Kuhling and Peters, 1994).
For the UK,
there is a distinct lack of available information on the dry and wet deposition
of arsenic, both at the local level and at the regional scale.
3.2
Vapour-phase Arsenic
Lahmann et
al (1986) highlight in their consideration of heavy metal pollution across
Europe, that there exists the possibility that a number of metals may occur
in the gaseous state as a result of high temperature volatilization. For
example, metallic mercury, as well as organic and inorganic mercury compounds,
are known to occur in the vapour phase, as well as being bound to particulates.
In general,
Lahmann et al report that vapour phase concentrations of metals and their
compounds are unlikely to be a problem in the atmosphere and pose little
in the way of danger to health. In particular, the issue of lead alkyl
compounds, which have previously made a significant contribution to air
pollution owing to their volatility, are no longer regarded as a problem
since their use in fuel additives in large quantities is now restricted.
However, it is possible that methylated species of arsenic (e.g. monomethylarsonic
acid (MMA) and dimethylarsinic acid (DMA)) may occur as a consequence of
chemical transformations in the atmosphere around certain industrial processes.
For arsenic,
arsine (AsH3) and arsenic trioxide (As2O3)
are known to occur in the vapour phase. Whether these compounds occur at
such high concentrations to warrant extra attention is uncertain. In any
case, it is likely that problems will only exist in the vicinity of industrial
processes in which these compounds are used. To date, there has been no
reporting of vapour phase concentrations of arsenic in the UK, or anywhere
else in Europe.
4
UK Sources of Arsenic
The following
section outlines the primary sources of particulate-bound arsenic emissions
in the UK, and reports on latest emissions estimates from the UK National
Atmospheric Emissions (UK NAEI) and the Environment Agency’s Pollution
Inventory.
4.1
Combustion Sources
By far the
most significant emission sources for arsenic in the UK (ca. 87%) are found
in the ‘combustion source’ category. This occurs primarily as a result
of the concentration of arsenic in coal which are known to vary from trace
to 1-2%.
Emissions of
arsenic from stationary combustion sources are only relevant for processes
where fuel burning results in a considerable ash residue. For example,
in coal-fired units, the fineness of grinding is the controlling parameter
- the level of ‘fly ash’ carried by the stack emissions and, consequently
the quantities of particulate-bound metals, is dependent upon this parameter.
In order that the residual products are dealt with accordingly, it is necessary
for the units to use dust separators. The quantities of heavy metals released
into the atmosphere is therefore also dependent upon the efficiency of
these dust separators. In contrast, where fuel oils are burnt, the level
of particulates in the emissions is dependent mainly on the efficiency
of combustion.
Commercial
and residential thermal generators have similar characteristics to larger
power generation units although, in general, lack the dust separators employed
by the larger units. Thus, where a large number of commercial or residential
thermal generators are present, the cumulative emissions of particulates
can be significant.
In all combustion
processes, arsenic is generally present in the flue gas as compounds (e.g.
oxides, chlorides, etc.) condensed onto the surface of particles. In addition,
it has been estimated that arsenic is emitted to a small extent in the
vapour phase - calculated as 0.5% (wt) from the arsenic content of coal
(USEPA, 1998).
Results for
the latest estimates of emissions of arsenic in the UK obtained from the
National Atmospheric Emissions Inventory (NAEI, 1997) indicate that, of
the top ten companies emitting arsenic to the atmosphere, eight of the
top ten positions are held by companies belonging to the power generation
sector (Table 4.1). The remaining two positions are held by companies belonging
to the mineral and metal production industrial sectors.
Table 4.1:
Top Ten Emitters of Arsenic in the UK, 1997 (Pollution Inventory, 1999)
| Company
Name, Postcode |
Industry |
Emissions
of Arsenic (tonnes) |
| Rugby
Group plc., DN18 6JL |
Mineral
Industries |
1.0000
|
| PowerGen
(UK) plc., ME3 9LY |
Fuel
and power generation |
0.8166
|
| Britannia
Zinc Ltd., BS11 8HT |
Metal
production |
0.7359
|
| PowerGen
(UK) plc., WF11 8SQ |
Fuel
and power generation |
0.5430
|
| PowerGen
(UK) plc., WA5 2UT |
Fuel
and power generation |
0.5140
|
| PowerGen
(UK) plc., DN22 0EU |
Fuel
and power generation |
0.4970
|
| National
Power plc., DN14 0UZ |
Fuel
and power generation |
0.4690
|
| National
Power Drax Ltd., YO8 8PJ |
Fuel
and power generation |
0.4090
|
| Eastern
Merchant Generation Ltd., DN22 9BL |
Fuel
and power generation |
0.3940
|
| Eastern
Merchant Generation Ltd., NG23 6SE |
Fuel
and power generation |
0.2400
|
4.2
Production Processes
Commercial
arsenic is primarily produced as a by-product in the smelting of non-ferrous
metal ores containing gold, silver, lead, nickel and cobalt. The amount
of arsenic found in lead and copper ores may range from a trace to 2 -
3 %.
The primary
uses of arsenic containing compounds in the UK today are as wood preservatives
and in the manufacture of chemical compounds (with arsenic trioxide (AsO3)
being the sole base material). In recent years, the use of refined arsenic
trioxide, used as a decoloriser and finishing agent in the manufacturing
of bottle glass and other types of glassware, has been replaced by arsenic
acid. In addition, there is also a limited demand for high-purity arsenic
(99.9% and greater) for use in the semiconductor and electronics industry
where it is used together with gallium or indium for producing light emitting
diodes (LED), infrared detectors, and lasers.
The following
sections consider some of the more relevant processes in more detail :
4.2.1
Non-ferrous Metal Industry
Smelting processes
are carried out at high temperatures and large quantities of dust and metal
oxides fumes can be generated as a result. It is known that a large number
of trace elements present in ores, and in concentrates, are volatilized
by the high temperatures used in processes such as roasting, syntering,
smelting and converting operations (Lahmann et al, 1986).
Small amounts
(around 0.5%) of arsenic are added to lead-antimony grid alloys, used in
lead acid batteries, to increase endurance and corrosion resistance. Additions
of the same order (0.02% to 0.5%) to copper alloys raise the re-crystallisation
temperature and improve high temperature stability and corrosion resistance
of the alloys. Other uses of metallic arsenic include the addition (up
to 2%) to lead in shot to improve the sphericity of lead ammunition.
Arsenic trioxide
is easily volatilized during the smelting of copper and lead concentrates,
and is known to become concentrated in the flue dust. Crude flue dust may
contain up to 30% arsenic trioxide. This crude flue dust is subsequently
upgraded by mixing with a small quantity of pyrite or galena and roasting
the subsequent mixture. During the roasting process the gases and vapours
are allowed to pass through a cooling flue which consists of a series of
brick chambers or rooms called ‘kitchens’. The arsenic vapour which condenses
in these chambers is of varying purity (from 90 to 95%). Higher purity
products can be obtained by resubliming the crude trioxide, an operation
typically carried out in a reverberatory furnace.
4.2.2 Iron & Steel Industry
Iron ores
contain a number of arsenic compounds that can be released as particle-bound
pollutants during different production processes from the iron and steel
industry. The exact profile of emitted metal compounds is dependent upon
the composition of the ores used and the individual production steps in
the iron and steel industry make varying contributions to arsenic levels
in the atmosphere. Most emissions of arsenic from the iron and steel industry
result from blast furnace charging where coarse dust (with particle sizes
> 10 m m) is found. Other relevant emissions occur in connection with melting
processes of arsenic-polluted scrap in electric arc furnaces.
4.2.3
Sinter Plants
Another relevant
source of emissions of arsenic in the UK is the sintering process, an ore
pre-treatment step in primary metals production where fine particles of
metal ores are agglomerated to briquettes, sinter or pellets. The whole
sintering process consists of several steps (e.g. mixing, crushing, sieving
and sintering) where emissions of metal-containing dusts can occur. Hutton
& Symon (1986) estimate that sinter production in the UK produced atmospheric
emissions of 7.5 tonnes arsenic per year in 1986.
4.2.4
Non-ferrous Metal Mining
Hutton and
Symon (1986) highlight that a large number of disused mines in the UK could
represent a potentially significant source of contamination of arsenic
at the local level through wind dispersal of material from unstable spoil
heaps at these sites. However, despite the widespread occurrence of such
heaps, it is currently not possible to estimate the quantities of elements
released into the atmosphere due to uncertainties in emissions and the
reliance on local meteorological conditions.
4.2.5 Waste Treatment and Disposal
Emissions
of arsenic from waste incinerators are wholly dependent upon the composition
of the waste, the combustion conditions and the clean technology applied
to the stack. Incineration is a complex combustion process involving several
key steps: pyrolitic decomposition; surface and gas combustion; conductive,
convective and radiative heat transference, and gas flow through beds of
materials whose quality, size and shape are continuously changing. Due
to the complex nature of the waste incineration process, it is difficult
for operators to define optimum conditions for operation and thus environmental
problems can result as a consequence of emissions. It has been shown that
urban incinerators can be an important source of airborne heavy metals
such as arsenic (Law & Gordon, 1979). In addition, waste sites have
been shown to be sources of methylated gaseous compounds (Feldmann et al.,
1994).
4.2.6
Timber Industry
The manufacture
of copper chromium arsenic (CCA) wood preservatives can lead to significant
emissions of arsenic to the UK atmosphere. For example, the disposal of
treated timber after use results in an estimated environmental discharge
of approximately 9 tonnes arsenic per year as a result of burning (Hutton
and Symon, 1986). Currently, a large quantity of CCA-treated timber is
still in service making it likely to remain a potentially significant emission
source in the future. This fact has been emphasised in a recent report
by WS Atkins in which the relevant risks associated with the use arsenic-based
wood preservatives, covering the manufacture of CCA and the production,
use and disposal of CCA-impregnated wood is discussed. In its appraisal
of the report, the Scientific Committee on Toxicity, Ecotoxicity and the
Environment (CSTEE), broadly accept the risk assessment methodology which
highlights that, in general, the risks most associated with the use of
CCA-impregnated wood are those resulting from the leaching of CCA into
water and soil. For emissions to air, the report indicates that it is the
disposal of CCA-impregnated wood that is likely to represent the largest
risk. In particular, uncontrolled burning of CCA-treated wood in homes
or on open ground.
4.3
Emission Inventory Estimates
The UK National
Atmospheric Emissions Inventory (NAEI) currently reports emissions of arsenic
on an annual basis. The reported figures cited in Table 4.2 and Figure
4.1 are quoted from the latest (1997) report. Results show that emissions
have declined by 78% since 1970.
In the UK,
the main source of arsenic emissions has been the combustion of fossil
fuel (notably coal), other sources being small in comparison. In part,
the reduction in UK emissions of arsenic over the past two decades has
resulted as a consequence of a decline in coal use, in favour of natural
gas use as a cheaper alternative.
In addition
to the NAEI emissions estimates of arsenic, the Environment Agency have
recently disseminated information regarding a wide variety of industrial
processes in England and Wales through the Pollution Inventory. In Scotland,
this duty falls to the Scottish Environment Protection Agency for which
currently no similar database of emitters is available. Thus, without detailed
scrutiny of the Public Registers, there is a lack of available information
reported in the current context.
The Pollution
Inventory has been developed to provide information on annual mass releases
of specified substances to air, water land or produced as waste which arise
from any large industrial sites (i.e. those authorised by the Environment
Agency under Integrated Pollution Control (IPC)). The reporting requirements
for the Pollution Inventory encompass emissions from the whole of the IPC
authorisation. That is, it includes, along with the specific release points
(point sources, e.g. chimneys), non-point sources and fugitive emissions
(e.g. leaks or spillages).
All authorisations
for large industrial sites (i.e. those under IPC) have a condition requiring
the annual reporting of releases of a "core" list of substances and groups
of substances These are substances considered to be important in relation
to environmental protection. By ensuring that all sites report, the Environment
Agency can be more confident that they are getting a complete picture of
what is being released nationally from large industrial sites.
The latest
(1998) Pollution Inventory estimates of arsenic emissions for large industrial
sites in England and Wales are summarised in Annex
1.
Figure
4.1 : Annual UK Emission of Arsenic (1970 - 1996) (NAEI)
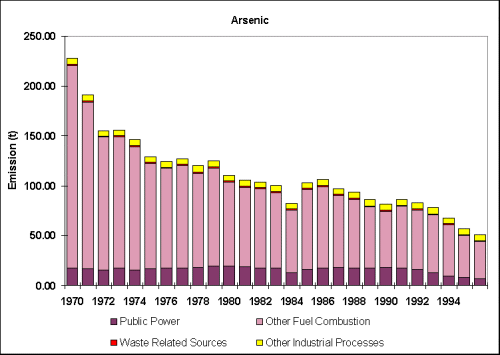
Results of
the NAEI confirm Pollution Inventory estimates on arsenic emissions in
the UK which indicate that the largest emission sources of arsenic in the
UK are associated with combustion processes related to the power generation
sector.
PowerGen, National
Power, and Eastern Merchant Generation power generating plants dominate
the top ten rankings. In addition to these power generators, other large
emission sources of arsenic in England and Wales include the Rugby Group
plc. plant on Humberside, and the Britannia Zinc Ltd. plant in Avonmouth,
Bristol. In 1998, arsenic emissions from these plants were estimated at
1.000 tonnes and 0.7359 tonnes, respectively.
Table 4.2
: UK Emissions of Arsenic by UNECE Category (tonnes) (NAEI, 1997).
| |
1970 |
1975 |
1980 |
1985 |
1990 |
1993 |
1994 |
1995 |
1996 |
| By
UNECE Category |
|
|
|
|
|
|
|
|
|
| Comb
in Energy Prod & Trans |
26 |
23 |
23 |
18 |
19 |
14 |
11 |
9 |
7 |
| Comb
in Comm/Inst/Res/Agri |
|
|
|
|
|
|
|
|
|
| Domestic |
88 |
51 |
39 |
38 |
19 |
20 |
17 |
12 |
12 |
| Other |
19 |
9.1 |
8.4 |
7.9 |
5.6 |
3.9 |
3.4 |
2.4 |
2.7 |
| Combustion
in Industry |
|
|
|
|
|
|
|
|
|
| Treated
Wood |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
9 |
| Iron
& Steel |
4.1 |
1.6 |
1.0 |
0.4 |
0.3 |
0.3 |
0.2 |
0.5 |
0.7 |
| Other
Combustion in Industry |
73 |
30 |
24 |
23 |
23 |
24 |
21 |
18 |
13 |
| Non-Ferrous
Metals |
2.9 |
2.5 |
3.1 |
3.2 |
3.2 |
3.4 |
3.3 |
3.0 |
3.2 |
| Cement
Manufacture |
0.2 |
0.2 |
0.2 |
0.1 |
0.2 |
0.1 |
0.1 |
0.1 |
0.1 |
| Glass
Production |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
| Production
Processes |
|
|
|
|
|
|
|
|
|
| Processes
in Industry |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
| Iron
& Steel |
2.4 |
1.9 |
1.2 |
1.6 |
1.8 |
1.7 |
1.7 |
1.7 |
1.8 |
| Extraction
and Distribution of Fossil Fuels |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
| Road
Transport |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
| Other
Transport & Machinery |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
| Waste
Treatment & Disposal |
1.1 |
1.1 |
1.1 |
1.1 |
0.9 |
0.8 |
0.6 |
0.5 |
0.3 |
| Total |
228 |
129 |
110 |
103 |
82 |
78 |
68 |
57 |
51 |
5
Air Quality Guidelines
At present,
there are no UK or EU guidelines set for arsenic in ambient air. Specifically
in the UK, limits have been set for large industrial processes under Integrated
Pollution Control (IPC) as part of the authorisation permitted by the Environment
Agency for the operation of such facilities in England and Wales. In Scotland,
corresponding duties are undertaken by the Scottish Environmental Protection
Agency (SEPA). However, these limits are set on the basis of best practice
and process control, rather than being set for the protection of human
health.
The lack of
ambient air quality guidelines in Europe for arsenic is, in part, due to
the fact that the likely levels of arsenic in air are, in general, low.
This fact highlights that exposure via air is less significant that other
routes, e.g. the consumption of foodstuffs and drinking water.
Currently,
the unit risk value cited in the update of the WHO Air Quality Guidelines
(WHO, in preparation) is 1.5 x 10-3 per mg m-3
of
pollutant, based upon observations of lung cancer in the population at
ambient concentrations of 1 - 30 mg m-3 x 10-3.
The unit risk
value has been given an International Agency for Research on Cancer (IARC)
classification of ‘1’ - that is, arsenic is a proven carcinogen.
6
Measurement Techniques
This section
gives a general overview of methods employed for the measurement of arsenic
concentrations in air in the UK. It outlines analytical methods employed
for the analysis of total suspended particulates. In addition, consideration
is given to the determination of vapour-phase arsenic.
6.1
Sampling
Conventional
measurement methods for arsenic typically involve a two-stage process whereby
samples are collected and then analysed at a later date.
A variety of
sampling and analytical techniques have been used to measure trace metal
concentrations in air which include impingers, electrostatic precipitators
and filters. Common features to these sampling and analysis techniques
employed in the UK over the last 15 - 20 years are:
-
total atmospheric
particulate samples are collected and analysed with no distinction between
particle sizes;
-
the duration of
sampling varies but typically spans one week (in the UK). The temporal
resolution thus tends to be less than for many other pollutants measured
in the UK with the consequence that ‘peak’ short-term concentrations may
pass unrecorded.
Over recent
years, filters have been favoured over other measurement methods due to
their high efficiency of collection of small particles. Specifically, sampling
of arsenic in the UK has been undertaken using the ‘M-Type’ sampler. This
sampler has been designed to measure airborne particulate matter in a size
range that is representative of respirable material and consists of two
distinct parts: a control box and a sample hood and stand. The flow rate
is controlled to 0.3 m3/h and material collected over a one-week
period onto Millipore Aerosol Field Monitor filters (mixed cellulose esters,
size 37 mm diameter, 0.8 micron retention).
6.2
Filter Media
Typically,
a number of different filter media have been considered for the determination
of trace metal concentrations in air. These include glass fibre filters
(borosilicate glass), quartz fibre filters, membrane filters and teflon
filters.
An important
consideration in selecting the most appropriate filter medium for the collection
of trace elements in particulate matter is the background metal concentration
of the filter material (the blank value). For example, Harrison (1986)
determined that the blank value of arsenic in glass fibre filters was 80
ng As cm-2. Teflon filters are generally considered to
be the best filter collecting medium for the determination of trace metal
concentrations due to the very low blank levels. Table 6.1 shows typical
properties of the filter types including blank values.
Table 6.1
: Properties of different filters types used for the collection of particulate
matter in air.
| Filter
Type |
Blank
value
ng As
cm-2 filter area |
Properties |
| Glass
Fibre |
40.0
- 60.0
|
Borosilicate
glass, blank values can be variable, low flow resistance. |
| Quartz
Fibre |
0.5
- 5.0
|
Quartz
glass, lower blank values for many elements compared to glass fibre filters,
similar flow resistance to glass fibre filters. Total digestion is easier
than glass fibre filters. Relatively expensive. |
| Membrane |
0.1
- 4.0
|
Cellulose
acetate or cellulose nitrate, low blank values, high flow resistance (increased
under high humidity conditions). Prone to electrostatic charge. |
| Teflon |
ca.
0.3
|
Very
low blank values. High flow resistance. Relatively expensive. |
For quality
assurance, field filter blanks should be included in any monitoring programme.
These filters should be handled in the same way as ‘normal’ filters but
without sucking ambient air through them. In addition, laboratory filter
blanks should be analysed.
6.3
Sample Preparation and analysis
In dust analysis,
the analytical step is generally preceeded by an appropriate preparation
of the sample in order to dissolve the collected particulate matter. This
dissolution can be achieved by either wet or dry procedures.
After dissolution,
analysis is carried out using a number of different techniques which vary
in their procedures and detection limits. They include; atomic absorption
spectrometry (AAS); inductively coupled plasma spectrometry (ICP); or,
X-ray fluorescence spectrometry (XRF).
Further details
of the sample preparation and the analytical techniques are given below:
6.3.1
Sample Preparation
In wet digestion
procedures, the sample is treated with a mixture of different acids, e.g.,
HCL, HNO3, HClO4 or HF, at normal or elevated pressure.
The choice of acid and digestion conditions depends upon the filter material
used to collect the sample and the element to be determined. In many cases,
digestion with hot nitric acid (with the addition of perchloric acid if
the sample has been collected on a membrane filter) has proved satisfactory.
Recent cross-comparisons
between sample dissolution methods (ultrasonic agitation, hot-plate digestion
and micro-wave assisted digestion procedures) indicate that variable results
can be obtained. For example, Butler & Howe (1999) report that ultrasonic
agitation digestion methods were satisfactory for most of the elements
with the exception of chromium and nickel which showed up to 30% lower
recovery values than micro-wave assisted digestion. For laboratories carrying
out digestions utilising hot-plates, Butler & Howe report the largest
amount of variation. Errors with this method were attributed to the fact
that the analyst is required to heat the samples to a ‘fuming’ end-point
which was open to interpretation. Insufficient or excessive ‘fuming’ were
likely to introduce errors through acid-matrix mismatches.
Small filters
(width up to 50 mm diameter) can be digested whole. For filters that are
larger than 50 mm, it is recommended that these filters be partitioned
into smaller pieces in order to reduce the amount of digestion solution
involved and to improve the homogeneity of the collection efficiency over
the surface of the complete filter area. If partitioning of filters is
used, it is necessary to ensure that sub-samples are representative of
the entire filter.
In dry pre-analysis
treatment, the sample is dry-ashed, preferably at low temperature (within
the range 100 - 150 oC). An advantage of this type of sample
preparation is that no reagents are required and hence, blank values can
be kept low. However, care is required in order to minimise losses of volatile
elements from the sample.
6.3.2
Commonly Used Analytical Methods
The following
section describes briefly the most commonly used analytical methods for
the determination of trace metal concentrations. It does not attempt to
produce a detailed review of the techniques which has been carried out
previously by a number of workers (e.g. see Harrison & Perry, 1977).
Atomic
Absorption Spectrometry (AAS)
AAS is probably
the most frequently used analytical method for the determination of particle-bound
heavy metals concentrations in air. In this technique, the analyte is aspirated
into an ionising flame that causes a chemical reduction of metal ions to
ground state atoms, the light absorption of which is measured. The atomic
absorptions are discrete lines of narrow band-width at wavelengths which
are characteristic of the given element. The wavelength range of AAS is
limited by light absorption by the flame that is used for the analysis.
For arsenic, an argon-hydrogen-entrained air flame is used with a typical
atomic absorption spectrophotometer such as a Perkin-Elmer. Typically,
the detection limits for arsenic quoted for the Perkin-Elmer spectrophotometer
are 0.05 m g ml-1 (double-beam instrument).
Inductively
Coupled Plasma / Mass Spectrometry (ICP-MS)
This technique
includes the following general procedures; the analyte solution is first
transferred into a pneumatic nebulizer to produce an aerosol. A stream
of argon carries this aerosol into the inductively coupled plasma formed
by ionising and exciting the inert argon gas. De-solvation, atomisation
and ionisation of the analyte occurs in the plasma with the resultant ions
separated in a vacuum on the basis of their mass-to-charge ratio by the
mass spectrometer. The ions transmitted through the mass spectrometer are
quantified by a pulse counting detector. Calibration with reference solutions,
based on the linear relationship between concentration and measured pulse
rate, allows a quantitative analysis of the sample analyte.
Analysis
of X-Ray Fluorescence (XRF)
This technique
relies on the principal that a metal target bombarded with X-rays results
in absorption and resultant secondary emission of X-rays (fluorescence)
characteristic to the irradiated metal. Using appropriate instrumentation,
the intensity of the secondary emissions may, by comparison with a suitable
standard, be used to give a quantitative measure of the metal. X-ray fluorescence
of particulate matter has the advantage over other analytical techniques
in that it is rapid, samples need no pre-treatment (i.e. digestion) and
it is non-destructive. Typical detection limits for X-ray fluorescence
analysis of particulate material collected on filter papers have been reported
by Harrison (1986). For arsenic, a value of 100 ng cm-2 for
arsenic has been cited, based upon energy dispersive analysis using radioisotopes
utilising a 10 minute count.
Neutron-Activation
Analysis (NAA)
NAA is a non-destructive
technique used in a number of studies to determine the elemental composition
of particulate matter (e.g. Dams et al., 1970). The sample is irradiated
with a flux of neutrons which induces the formation of isotopes emitting
a characteristic radiation. Specific detectors characterise the individual
elements. Although NAA is a relatively sensitive analytical technique,
some elements have been shown to be difficult to analyse (e.g. Pb and Cr),
and should therefore be determined using other methods.
6.4
Vapour-Phase Arsenic
A recent update
to the Health and Safety Executive’s (HSE’s) guideline document Methods
for the Determination of Hazardous Substances series MDHS 41 ‘ Arsenic
and inorganic compounds of arsenic in air’, includes the determination
of vapour phase arsenic (as arsenic trioxide) in the workplace where exposure
of individuals to arsenic may occur during the manufacture and use of arsenic
compounds.
The procedure
relies on the collection of arsenic compounds (particulate and vapour (only
arsenic trioxide; arsine is not trapped)) by drawing air through a two-stage
sampling unit containing two filters. The first, a cellulose ester membrane
filter captures particulate arsenic and inorganic arsenic compounds whilst
the second, a sodium carbonate impregnated back-up paper pad, captures
arsenic trioxide vapour. This vapour is collected by the reaction with
sodium carbonate on the impregnated back-up filter pad through the following
stoichiometric relationship:
As2O3
+ Na2CO3  2NaAsO2 + CO2
2NaAsO2 + CO2
Analysis of
the cellulose ester membrane and sodium carbonate back-up pads is carried
out using atomic absorption spectrometry (AAS), This requires the initial
digestion of the filters with an acid mixture containing nitric acid, sulphuric
acid and hydrogen peroxide. Further details of the AAS method are given
above.
6.5
Proposed Sampling Method
The EC Working
Group for metals has recommended that the reference method for the determination
of ambient metals in air be based upon the Low Volume Sampler (LVS) as
described in EN 12341 (CEN, 1998). A low metal background filter material
(quartz), and total digestion and analysis with AAS-technology is recommended
by the Working Group. Other methods can be used, if their equivalence has
been demonstrated.
CEN/TC 264/WG
14 is currently formulating the full Reference Method for the determination
of Pb, Cd, As and Ni in ambient air, which will take note of the EC group
recommendation. A laboratory and field validation programme is currently
being formulated and will contain the following elements:
-
Sampling of particulates
using the LVS (described in EN 12341) for 24-hour sampling periods
-
Collection of
particulates on quartz fibre and membrane filters (not Teflon)
-
Total digestion
with and without HF (digestion procedure to be investigated using Certified
Reference Material filters).
-
Mandatory measurement
with AAS, with optional measurements using ICP-MS.
To date,
there has been a paucity in monitoring data for arsenic and other trace
elements in ambient air within the UK. The adoption of the common procedures
and the recommendations of the CEN Working Group will greatly increase
the level of confidence in the results obtained from future monitoring
programmes.
7
Monitoring in the UK
The following
section reviews airborne concentrations of arsenic in the UK. It details
the results of monitoring programmes that have been carried out under both
the auspices of the national monitoring network and also individual ad-hoc
studies carried out by private institutions and universities published
in scientific journals and/or reviews.
7.1
Rural Network Data
Four long-term
rural background sites have been monitoring particulate arsenic levels
since 1972; Chilton (Oxfordshire), Styrrup (Nottinghamshire), Windermere
(Wraymires, Cumbria) and Trebanos (West Glamorgan). The annual mean concentrations
for 1970 to 1998 are shown in Figure 7.1.
Figure 7.1
Annual mean concentrations of arsenic at rural monitoring locations in
the UK (1970 - 1998).
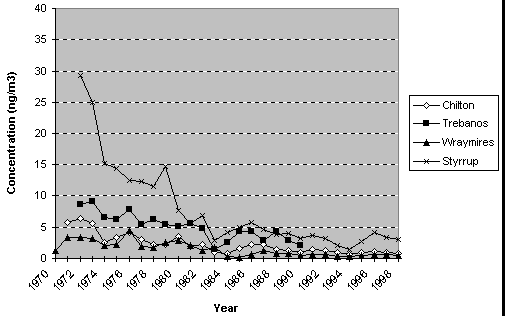
Results show
a decline in the ambient levels of arsenic in rural areas throughout the
UK. This decline is more prominent at the Styrrup site where ambient concentrations
have declined from around 30 ng m-3 in 1972 to around 3 ng m-3
in 1998. The higher concentrations of arsenic reported at this site, compared
to concentrations recorded during the same period (1972 - 1981) for other
sites, are likely to be the result of emissions from coal-fired power stations.
These occur at a much higher frequency in The Midlands than in other parts
of the UK.
Recent analysis
of the data obtained from these sites has been presented (Cawes et al,
1994) with the long-term trends having been analysed over the last two
decades (covering 1972-1981 and 1982-1991). Results are summarised in Table
7.1.
Table 7.1
: Rural concentrations of arsenic in particulate matter in the UK (1972
- 1991) from Cawes et al, 1994.
| Site |
Concentration
(ng m-3)
|
| |
Mean |
Trend |
Mean |
Trend |
| |
1972
- 1981 |
%/year |
1982
- 1991 |
%/year |
| |
|
|
|
|
| Harwell,
Oxfordshire |
4
|
-10.0
|
1.7
|
-7.8
|
| Styrrup,
Nottinghamshire |
15
|
-14.3
|
4.4
|
-5.2
|
| Trebanos,
Glamorgan |
7
|
-6.0
|
3.5
|
-
|
| Wraymires,
Cumbria |
3
|
no
sig. trend
|
0.8
|
-7.3
|
The highest
arsenic concentration over the period 1972-1981 (15 ng m-3)
was found at the site in Nottinghamshire, whilst the second highest concentration
(7 ng m-3), over the same period, was found at the site in West
Glamorgan. In contrast, sites in Oxfordshire and Cumbria showed the lowest
concentrations of arsenic, 3-4 ng m-3, during the same period.
Results presented
by Cawes et al (1994) showed lower concentrations of arsenic were observed
at all sites covering the period 1982-1991. A similar picture in the ranking
of sites with respect to the concentrations was found: sites in Nottinghamshire
and West Glamorgan showed the highest concentrations of arsenic with sites
in Oxfordshire and Cumbria showing the lower concentrations.
Results from
the rural network highlight that ambient concentrations of arsenic in the
UK have been steadily declining over the last two decades. This rate of
decline has been generally higher in the first decade of monitoring (1972-1981)
compared to results of the monitoring carried out for the period 1982-1991.
This can be attributed to improvements to abatement technology and the
availability of natural gas as a cheaper alternative to traditional coal-burning
in the power generation sector.
7.2
Urban Network Data
In addition
to the four rural monitoring stations, arsenic in particulate matter has
been monitored at a number of urban and residential sites throughout the
UK from the mid 1970’s to the late 1980’s. Results for this network have
been recently published by Lee et al (1994) and previously reviewed by
QUARG (1993), and are shown in Figure 7.2 and are summarised in Table 7.2.
Figure 7.2
Annual mean concentrations of arsenic at urban monitoring locations in
the UK (1975 - 1990).
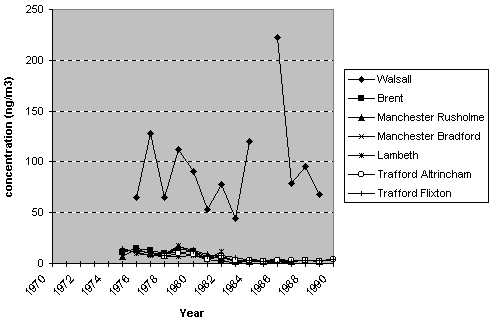
Results of
the annual mean urban concentrations show the highest annual mean concentration
of arsenic (223 ng m-3) occurred at an industrial site in Walsall
in 1986. This site is in the vicinity of a smelter and has continually
recorded the highest concentration of arsenic at any site in the UK since
monitoring commenced in the mid 1970s. Annual mean concentrations recorded
at other urban monitoring locations show similar values of around 10-15
ng m-3 in the mid 1970s to values around 5 ng m-3
in the early 1990s when monitoring ceased.
Analysis of
data by Lee et al (1994) has reflect the trends observed in the annual
mean data. The highest mean concentrations for the period covering mid
1970s to the late 1980s was recorded at Walsall (93 ng m-3),
whilst the second highest mean concentration over the period of monitoring
was recorded at the urban site in Swansea (19.0 ng m-3). Results
reported by Lee at al (1994) indicate that concentrations of arsenic in
residential areas are typically in the region of 5 - 7 ng m-3,
approximately 3-4 times higher than those observed at rural monitoring
locations.
The seasonal
variability of arsenic has been examined by Lee et al (1994) over all urban
sites (except Walsall where a single dominant source was known to influence
concentrations). This was carried out through determining the ratio of
the quarterly mean to the annual mean concentration. In general, higher
concentrations of arsenic were found in the first quarter of the year when
compared to any other period - a ratio to mean just above 1.4, whilst concentrations
of arsenic were very similar in the second and fourth quarters of the year
showing a ratio to mean of approximately 0.9. The lowest arsenic concentrations
were observed during the third quarter of the year where a quarter mean
ratio to annual mean value of approximately 0.7 was reported. Lee et al
(1994) offer no explanation for the seasonal variability in this element
although it can be hypothesised that the seasonal variability may be explained
in terms of its predominant source, coal and fuel-oil combustion. The higher
concentrations in the first quarter, when demand for heating and electricity
is greater, support this hypothesis. The seasonal variability in concentrations
highlights the fact that anomalous results in monitoring programmes will
be obtained with respect to the determination of annual mean concentrations
where the observation period are based on a short duration, e.g. 3 months
in the winter.
Table 7.2
: Urban Concentrations of Arsenic in the UK (1972 - 1989) from Lee et al.
(1994).
| Location |
Site
Classification |
Period |
Mean
(ng
m-3) |
Min
(ng
m-3) |
Max
(ng
m-3) |
| |
|
|
|
|
|
| Trafford
- Altrincham |
Residential |
1978
- 89 |
4.7
|
0.4
|
17.6
|
| London-
Brent |
Residential |
1975
- 89 |
6.1
|
0.2
|
24.3
|
| Trafford
- Flixton |
Residential |
1975
- 89 |
7.1
|
0.4
|
30.6
|
| London
- Lambeth |
Residential |
1976
- 82 |
7.2
|
0.8
|
15.3
|
| Manchester
City North |
Industrial
/
Residential |
1975
- 88 |
7.6
|
0.2
|
43.0
|
| Manchester
City South |
Residential |
1975
- 89 |
6.5
|
0.2
|
41.0
|
| Swansea |
Urban |
1972
- 81 |
19.0
|
|
|
| Walsall |
Industrial |
1976
- 89 |
93.9
|
10.6
|
572.3
|
Long-term trend
analysis carried out by Lee et al (1994) on arsenic concentrations in the
UK indicate a decline in concentration across all urban sites (excluding
Walsall) over the last two decades. For example, the mean arsenic concentration
for 1975-1978 was 10.3 ng m-3 which fell by 74% to a concentration
of 2.7 ng m-3 for the period 1986-1989. Lee et al (1994) highlight
the decline in domestic emissions of coal covered by the Clean Air Act
(1956), as the possible leading factor in the decrease of ambient levels
of arsenic in recent years.
7.3
North-Sea Programme
In addition
to the rural and urban monitoring stations within the UK, monitoring for
arsenic has been carried out at a small number of stations in relation
to the North Sea Project of the Natural Environment Research Council.
The Humber
Estuary receives significant inputs of arsenic as a result of the relatively
dense industrial units located along the River Trent (Millward et al.,
1997). Increased arsenic concentrations (of DMA (dimethylarsenic)) in estuarine
waters have been shown to occur as far afield as the Wash in Norfolk as
a result of atmospheric enrichment from industrial processes located on
the Humber estuary (Millward et al., 1997).
In addition
to the number of estuarine sites reported by Millward et al. (1997), three
land sites have been included in the study at East Ruston (Norfolk), High
Muffles (Yorkshire) and Banchory (Kincardinshire). Results for the monitoring
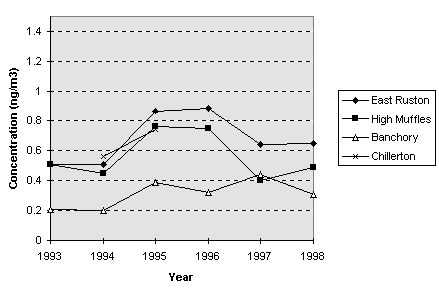
period covering 1993 - 1998 are shown in Figure 7.3.
Results show
similar concentrations to those observed at rural monitoring locations
elsewhere. That is, annual average concentrations of arsenic of around
0.5 to 1.0 ng m-3.
Figure 7.3
Annual mean concentrations of arsenic at land sites included in the North-Sea
monitoring programme (1993 - 1998).
8
Conclusions
Particulate-phased
arsenic concentrations have been monitored in the UK at a number of sites
covering rural (Cawes, 1994) and urban locations (Lee et al., 1994) for
the past two decades. Results show that concentrations around individual
industrial installations can be high (572 ng m-3 - 3 month mean
at Walsall (as cited in Lee et al., 1994)). In general, urban concentrations
of particulate-phased arsenic fall within the range 5 - 7 ng m-3
with rural locations falling within the range 0.8 - 4.5 ng m-3.
Currently,
no air quality standard exists in the UK for arsenic in air. Arsenic is
a known carcinogen and the World Health Organisation (WHO, 1987) acknowledges
that there is no acceptable limit to be set. However, health risk assessments
have been made which report a unit risk value of 1.5 x 10-3
for each m g m-3 of arsenic to which an individual is exposed
(WHO, 1987).
Emissions estimates
for arsenic obtained from both ad-hoc studies and the UK National Air Emissions
Inventory (NAEI) indicate that combustion of fossil fuels such as lignite
(brown coal), hard coal and heating oil account for the largest proportion
of total UK emissions of arsenic. Other relevant emission sources of arsenic
to air in the UK include ferrous and non-ferrous production processes.
In each case, the emissions of arsenic to air are largely dependent upon
the arsenic content of the metals ore and/or fossil fuel and the propensity
of the process to produce particulate emissions. Emissions from the chemical,
glass and cement industries are generally much smaller than those from
other industrial sectors of the UK.
Trend analysis
of ambient concentrations of arsenic in the UK indicate that concentrations
have been steadily decreasing over the 20 or so years that monitoring has
taken place. Such a trend is likely to reflect the advances in clean technology
and pollutant abatement schemes (e.g. flue gas desulphurisation (FGD) and
electrostatic precipitators (ESP)) integrated as a condition of the authorisation
for many of the processes from which arsenic is known to be emitted. In
addition, the switch from traditional coal-burning to natural gas as a
cheap alternative in the power generation sector are likely to have made
the largest significant decrease in emissions of arsenic to air in recent
years.
The present
review highlights a number of gaps in the current knowledge of arsenic
occurrence in the UK. In particular, any detailed monitoring in Scotalnd
and Wales. Notably, there has been little work carried out on identifying
different species of arsenic in the atmosphere - to date, all ambient monitoring
of arsenic carried out has reported total arsenic concentrations in the
particulate phase. The review highlights that more consideration is required
to determine vapour-phase concentrations (as arsenic trioxide (AsO3))
of arsenic, but acknowledges that this is likely to be a problem with regards
to occupational exposure only.
In addition,
the review highlights that a cohesive approach to the monitoring of arsenic
is required within the UK in which monitoring methods are standardised
with respect to the particulate fraction to be collected. Traditionally,
the monitoring of arsenic has been made on the total suspended particulates
in air. Recently, an increased understanding of the occurrence of particulates
in air highlight the importance of the fine (PM10) and ultra-fine
(PM2.5) fraction (APEG, 1999).
The first Daughter
Directive published in 1997 by the European Commission reached the common
position in June 1998 for a number of pollutants with the proposals to
set limit values for sulphur dioxide, nitrogen dioxide, particulate and
lead. The Commission intends that future Daughter Directives will set limit
values for a number of other pollutants for which arsenic (among other
heavy metals) has been included. Currently, the Department of the Environment,
Transport and the Regions is funding consultants, Stanger Science and Environment
to undertake 12 months monitoring of lead and heavy metal concentrations
in response to these Commission’s proposals. In light of the recent review
of the National Air Quality Strategy (HMSO, 1999) the main focus of attention
in the 12 month monitoring period is in the vicinity of industrial processes.
Results are likely to be made available in December 2000.
References
APEG (1999).
Source Apportionment of Airborne Particulate Matter in the United Kingdom.
Report of the Airborne Particles Expert Group. January 1999.
Becher, H.
& Wahrendorf, J. (1992). Metalle/Arsen. In: H.E. Wichmann, H.W. Schlipkoter,
G. Fulgraff: Handbuch Umweltmedizin: Toxikologie, Epidemiologie, Hygiene,
Belastungen, Wirungen, Diagnostik, Prophylaxe, Ecomed Verlag, Landsberg,
Kap. VI-3.
Butler, O.T.
& Howe, A.M. (1999). Development if an international standard for the
determination of metals and metalloids in workplace air using ICP-AES:
evaluation of sample dissolution procedures through an interlaboratory
trial. J. Environ. Monit., 1, 23-32.
Cawes, P.A.,
Baker, S.J., & Law, D.V. (1994). A survey of atmospheric trace elements
in Great Britain, 1972-1991. Report number: AEA/CS/18358008/REMA-039. AEA
Technology, National Environmental Technology Centre, Culham Laboratory,
Oxfordshire.
CEN (1998).
Air Quality - Determination of the PM10 fraction of suspended
particulate matter - Reference method and field test procedure to demonstrate
reference equivalence of measurement methods. European Committee for Standardization
(CEN). Ref. No. prEN 12341: 1998 E.
Dams, R., Robbins,
J.A., Rahm, K.A. and Winchester, J.W. (1970). Non-destructive neutron activation
analysis of air pollution particulates. Anal. Chem., 42, 861.
Feldmann, J.,
Grumping, R., & Hirner, A.V. ( 1994). Determination of volatile metal
and metalloid compounds in gases from domestic waste disposits with GP/ICP-MS.
J. Anal. Chem., 350, 228-234.
Harrison, R.M.(1986).
Metal analysis. In: Handbook of Air Pollution Analysis. R.M. Harrison
and R. Perry (Eds.). Pub. by Chapman and Hall, Ltd. University Press, Cambridge.
HMSO (1999).
Review of the United Kingdom National Air Quality Strategy: A Consultation
Document. Published by the Department of the Environment, Transport and
the Regions in partnership with the Welsh Office, Scottish Office and Department
for the Environment for Northern Ireland, HMSO.
HSE (1995).
Arsenic and inorganic compounds of arsenic in air. Part of the HSE’s Methods
for the Determination of Hazardous Substances (MDHS 41/2.) Series. Published
by the Health and Safety Executive.
Hutton, M and
Symon, C. (1986). The quantities of cadmium, lead, mercury and arsenic
entering the UK environment from human activities. The Science of the
Total Environment, 57, 129-150.
Lahman, E.,
Munari, S., Amicarelli, V., Abbaticchio, P., & Gabellieri, R. (1986).
Environment and Quality of Life. Heavy Metals: Identification of air quality
problems and environmental problems in the European Community. Volumes
1 and 2. Commission of the European Communities Report Number EUR 10678,
CEC, 1987.
Law, S.L. &
Gordon, G.E. (1979). Sources of metals in a municipal incinerators emissions.
Environ.
Sci. Technology., 13, (4), 432 - 437.
Lee, D.S.,
Garland, J.A. and Fox, A.A. (1994). Atmospheric concentrations of trace
elements in urban areas of the United Kingdom. Atmospheric Environment,
28, 2691-2713.
Ledd, D.S.,
Espenhahn, S.E., and Baker, S. (1998). Evidence for long-term changes
in base cations in the atmospheric aerosol. Journal of Geophysical Research
103, 21955 - 21966.
Millward, G.E.,
Kitts, H.J., Ebdon, L., Allen, J.I., and Morris, A.W. (1997). Arsenic species
in the Humber Plume, UK. Continental Shelf Research, 17 (4), pp. 435-454.
NETCEN (1998).
Air
Pollution in the UK . A report produced for the Department of
the Environment, Transport and the Regions. National Environmental Technology
Centre, Culham Laboratory, Oxfordshire.
Pollution Inventory.
Environment Agency website address:
http://193.122.103.90/wiyby/html/introduction.htm
QUARG (1993).
Urban Air Quality in the United Kingdom. First Report of the Quality of
Urban Air Review Group. January 1993. Department of the Environment.
Slooff, W.,
Haring, B.J.A., Hesse, J.M., .Janu, J.A., and Thomas, R. (1990). Integrated
Criteria Document Arsenic. Report No 7104001004.
United Kingdom
National Emissions Inventory -
/netcen/airqual/naei/annreport/annrep96/sect6_3.htm
USEPA (1998).
Locating and Estimating Air Emissions from Sources of Arsenic and Arsenic
Compounds, USEPA, Office of Air Quality Planning and Standards, EPA-454/R-98-013.
WHO (1987).
Air Quality Guidelines for Europe. World Health Organisation Regional Office
for Europe, Copenhagen. WHO Regional Publications. European Series 23.
WHO (in preparation).
Air Quality Guidelines for Europe, 2nd edition. World Health Organisation,
Regional Office for Europe, Copenhagen, WHO Regional Publications, European
Series, Internet address: http://www.who.dk
Report prepared by Stanger Science and Environment. Site prepared by the National Environmental Technology Centre, part of AEA Technology, on behalf of the UK Department of the Environment, Transport and the Regions



