Base Cations
Introduction
Base cation emission estimates for the UK are presented in this chapter. The emission estimates cover the period 1990-2000 for Calcium (Ca), Magnesium (Mg), Sodium (Na) and Potassium (K). These estimates are very uncertain.
A base cation is essentially a positively charged ion from group 1 or 2 of the periodic table (the alkali metals or alkaline earth metals). The most environmentally abundant of these are Na, K, Ca and Mg. Base cations are important in the environment because their deposition has an impact on the surface pH. The deposition of base cations increases the alkalinity of the surface, the effect in the environment is to buffer or neutralise the effects of the acidity generated by S an N deposition (which in their mobile anionic form as SO42- and NO3- leach Ca and Mg from the soil). Therefore the primary purpose of these emission estimates is to use them to generate spatially resolved emission maps, which enable deposition maps to be calculated.
Background
A “critical load” approach is often taken to predict the maximum levels of acidity or alkalinity that an ecosystem can tolerate. The base cations (Na+, K+, Ca2+, Mg2+) are known to be present in ambient air and in precipitation. The deposition of these base cations to ecosystems will offset the acidifying inputs from the deposition of SOx and NOx. Acidifying emissions of SOx and NOx have already been quantified in the NAEI, and the effects of these acidifying emissions can now be complemented with estimates of emissions of important base cations.
The Review Group on Acid Rain (1997) reported on the decline in base cation deposition that has been observed in Europe and N America since the early 1970’s and how such a decline may offset the benefits of reductions in SO2 emissions. Interest in the deposition and acid neutralising effects of base cations is mainly confined to Ca, K and Mg. It has long been assumed that the major source of these base cations in air is dust from soil erosion, but patterns of concentrations in air and precipitation also suggest significant emissions from urban and industrial sources. The concentrations of Ca, K and Mg in air and in precipitation measured at three rural sites in the UK declined dramatically between 1970 and 1991 (Lee and Pacyna, 1999). It has been suggested that the decrease in base cation deposition which has been observed is due to the reduction in emissions from urban and industrial sources. Concentrations of Na in air and rain have shown much smaller decreases over this period, consistent with its mainly marine origin as sea-salt.
The NAEI has attempted to estimate emissions from the following sources:
· Stationary combustion of fossil fuels: mainly in the fly ash from solid fuel combustion
· Mineral extraction processes: e.g. limestone quarrying
· Processes in the mineral products industry: e.g. cement manufacture and concrete batching
· Industrial processes using limestone, dolomite and soda lime:
- iron and steel manufacture
- glass manufacture
·
Agricultural use:
e.g. liming of soils and dust due to cultivation.
·
Construction and
demolition activities
· Mobile sources: mostly in the form of dust resuspension by traffic and exhaust emissions of potassium from lead replacement petrol (LRP).
There are likely to be base cation emissions from other sources, for example incineration. Currently, these are not included in the estimates as such sources are likely to be much smaller than the sources listed above.
Stationary combustion of fossil fuels
The base cations emitted from stationary combustion arise from the trace concentrations of the cations found in the fuels. The base cations will enter the atmosphere contained in the primary particulate matter (PM) which is emitted from the combustion source. Calcium has been found in large amounts in the fine particle size fraction collected from combustion sources.
The NAEI currently estimates PM10 emissions from large combustion plant for power generation using total PM emissions data submitted by the operators to the Environment Agency and the Scottish Environmental Protection Agency. Where reported data are incomplete, PM emission factors for the appropriate fuel are derived and combined with the amount of fuel used by the combustion plant to estimate the total mass of PM emitted.
The mass content of cations in coal has been estimated from the Turner-Fairbank Highway Research Centre (US Transport Department) figures for fly ash from bituminous coal. Data regarding the composition of fuel oil is given in the Marine Exhaust Research Programme.
Mineral extraction processes
Limestone quarrying is a major source of atmospheric emissions of base cations, principally calcium. Quarrying of dolomite (CaCO3 MgCO3), rock salt (NaCl) and potash (KCl) are the principle sources of magnesium, sodium and potassium respectively.
The NAEI currently estimates PM10 emissions from quarrying using USEPA emission factors combined with UK mineral statistics on the production of each type of aggregate. The dust emitted from limestone quarrying will be mainly particles of limestone (CaCO3) itself. These particulates will be mainly in the coarse particle size range (>2.5 mm) and will be deposited close to their source. The quantities of these minerals extracted in the UK are given in the Minerals Yearbook (1990 – 2000).
Processes in the mineral products industry
Emissions of calcium from the mineral products industry are estimated from total PM10 emissions using emission factors from Lee and Pacyna (1999) or AEAT estimates of PM10 composition.
Industrial processes using limestone, dolomite and soda ash
Processes involving limestone, dolomite and soda ash include iron and steel production and glass manufacturing. Emissions of base cations from the iron and steel industry and the glass industry are based on the PM10 inventory combined with emission factors for cations taken from Lee and Pacyna (1999) or based on AEAT estimates of PM10 composition.
Soil liming and cultivation in agriculture
The practice of soil liming in agriculture will lead to the emission of Ca as the lime is applied to the ground. Statistics are available on the quantity of limestone used each year for liming (UK Minerals Yearbook) and an emission is estimated using an emission factor for non-metallic particles given by the USEPA.
The average quantities of re-suspended dust, as a result of land cultivation, may be estimated from data reported in the MAFF Report CSG 15 (2000). Emissions are estimated from the average chemical abundance of each cation in UK soil (Lindsay, 1979).
Construction activities
The NAEI currently uses a USEPA emission factor combined with UK construction activity statistics to estimate fugitive emissions of PM10 from these processes. A modified PM10 emission factor based on the fraction of total aggregate used in construction (UK Minerals Yearbook) that is limestone, dolomite or chalk, is used to estimate the base cation emissions.
Mobile sources
Emissions of base cations from mobile sources will mainly arise from the resuspension of road dust by traffic. Recently, Nicholson (2000) has made an estimate of the total PM10 emission from UK roads. Using this information with data on the average chemical composition of road dust (Sloss and Smith, 2000) Na, K and Ca emissions have been estimated. There are insignificant quantities of Mg in road dust.
Potassium compounds are the primary additives in Lead Replacement Petrol (LRP). LRP has been available since Autumn 1999 and is the main source of potassium emissions from vehicle exhausts. Emissions have been estimated from UK LRP sales in 1999 (calculated as a fraction of leaded petrol sales) and 2000 given by DUKES.
Table 7.1 Summary of Calcium Emissions in the UK (tonnes)
|
|
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
|
Comb. in Energy
Prod |
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
3211 |
3187 |
2989 |
2488 |
2210 |
1681 |
1548 |
1040 |
1077 |
844 |
952 |
|
Petroleum Refining Plants |
6 |
7 |
7 |
7 |
8 |
7 |
7 |
7 |
7 |
6 |
4 |
|
Other Comb. & Trans. |
14 |
13 |
9 |
6 |
3 |
1 |
1 |
1 |
1 |
1 |
1 |
|
Comb. in Comm./Inst/ Res |
|
|
|
|
|
|
|
|
|
|
|
|
Residential Plant |
1679 |
1801 |
1605 |
1640 |
1264 |
850 |
883 |
854 |
850 |
941 |
640 |
|
Comm., Public & Agri. |
147 |
138 |
116 |
102 |
90 |
66 |
72 |
80 |
52 |
46 |
38 |
|
Combustion in
Industry |
|
|
|
|
|
|
|
|
|
|
|
|
Iron & Steel Combustion |
3 |
3 |
3 |
4 |
4 |
3 |
2 |
2 |
2 |
3 |
1 |
|
Glass Production |
109 |
101 |
108 |
110 |
106 |
106 |
80 |
75 |
58 |
45 |
41 |
|
Other Comb. in Industry |
1542 |
1509 |
1559 |
1529 |
1477 |
1530 |
1428 |
1447 |
1336 |
1227 |
1051 |
|
Production Processes |
4424 |
4389 |
4091 |
4209 |
4655 |
4287 |
3985 |
4087 |
4086 |
3889 |
3786 |
|
Shipping |
8 |
8 |
8 |
8 |
7 |
8 |
9 |
11 |
10 |
7 |
6 |
|
Other Transp &
Mach. |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
0 |
0 |
0 |
0 |
|
Waste Treatment
& Disp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
|
Non livestock
agriculture |
269 |
84 |
84 |
84 |
76 |
76 |
177 |
177 |
42 |
34 |
34 |
|
Total |
11413 |
11242 |
10579 |
10187 |
9899 |
8615 |
8192 |
7783 |
7524 |
7045 |
6556 |
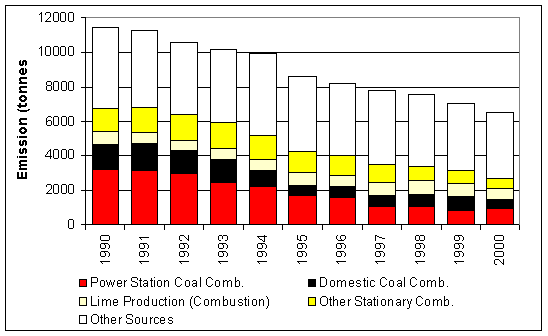
Figure 7.1 UK Emissions of Calcium
Table 7.2 Summary of Magnesium Emissions in the UK (tonnes)
|
|
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
|
Comb. in Energy
Prod |
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
1035 |
1029 |
966 |
804 |
715 |
543 |
500 |
337 |
349 |
273 |
308 |
|
Other Comb. & Trans. |
4 |
4 |
3 |
2 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Comb. in Comm./Inst/ Res |
|
|
|
|
|
|
|
|
|
|
|
|
Residential Plant |
543 |
583 |
519 |
531 |
409 |
274 |
285 |
276 |
275 |
304 |
206 |
|
Comm., Public & Agri. |
46 |
44 |
36 |
32 |
28 |
20 |
22 |
12 |
16 |
14 |
12 |
|
Combustion in
Industry |
|
|
|
|
|
|
|
|
|
|
|
|
Iron & Steel Combustion |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
3 |
|
Processes in Industry |
53 |
45 |
43 |
38 |
45 |
48 |
44 |
44 |
44 |
42 |
42 |
|
Glass Production |
55 |
53 |
54 |
54 |
54 |
59 |
40 |
40 |
30 |
23 |
22 |
|
Other Comb. in Industry |
225 |
245 |
280 |
258 |
241 |
225 |
191 |
174 |
134 |
123 |
87 |
|
Production
Processes |
551 |
546 |
532 |
523 |
517 |
376 |
354 |
360 |
329 |
286 |
277 |
|
Waste Treatment
& Disp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Non livestock
agriculture |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Total |
2517 |
2553 |
2436 |
2245 |
2012 |
1549 |
1439 |
1244 |
1180 |
1069 |
958 |
Figure 7.2 UK Emissions of Magnesium
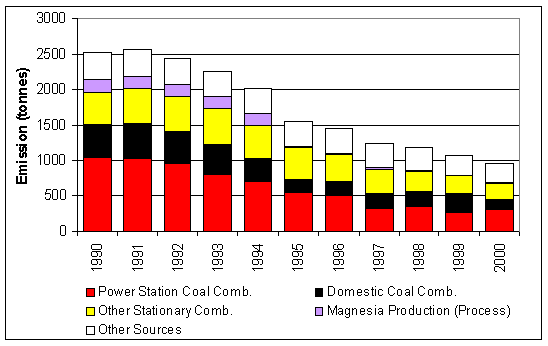
Table 7.3 Summary of Sodium Emissions in the UK (tonnes)
|
|
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
|
Comb. in Energy
Prod |
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
1032 |
1024 |
959 |
798 |
708 |
539 |
497 |
333 |
345 |
271 |
305 |
|
Petroleum Refining Plants |
4 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
4 |
3 |
|
Other Comb. & Trans. |
4 |
4 |
3 |
2 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Comb. in Comm./Inst/ Res |
|
|
|
|
|
|
|
|
|
|
|
|
Residential Plant |
542 |
581 |
519 |
530 |
410 |
278 |
289 |
280 |
278 |
307 |
211 |
|
Comm., Public & Agri. |
50 |
47 |
40 |
36 |
32 |
24 |
26 |
14 |
19 |
17 |
14 |
|
Combustion in Industry |
|
|
|
|
|
|
|
|
|
|
|
|
Iron & Steel Combustion |
308 |
298 |
288 |
287 |
295 |
302 |
317 |
322 |
314 |
300 |
268 |
|
Processes in Industry |
18 |
15 |
14 |
12 |
15 |
15 |
14 |
14 |
14 |
13 |
14 |
|
Glass Production |
113 |
102 |
111 |
113 |
108 |
101 |
86 |
78 |
64 |
52 |
46 |
|
Other Comb. in Industry |
229 |
250 |
284 |
262 |
245 |
227 |
193 |
175 |
135 |
122 |
87 |
|
Production
Processes |
127 |
144 |
135 |
128 |
148 |
152 |
167 |
152 |
103 |
124 |
130 |
|
Other Transp &
Mach. |
6 |
5 |
5 |
6 |
5 |
6 |
6 |
7 |
7 |
5 |
4 |
|
Non livestock
agriculture |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Waste Treatment
& Disp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Total |
2433 |
2475 |
2363 |
2179 |
1972 |
1651 |
1599 |
1380 |
1286 |
1216 |
1083 |
Figure 7.3 UK Emissions of Sodium
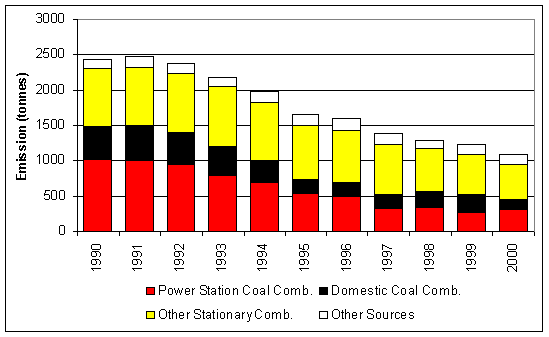
Table 7.4 Summary of Potassium Emissions in the UK
|
|
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
|
Comb. in Energy
Prod |
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
855 |
850 |
798 |
664 |
590 |
449 |
413 |
278 |
288 |
226 |
254 |
|
Other Comb. & Trans. |
0 |
3 |
2 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Comb. in Comm./Inst/ Res |
|
|
|
|
|
|
|
|
|
|
|
|
Residential Plant |
482 |
515 |
468 |
477 |
376 |
265 |
274 |
267 |
266 |
290 |
209 |
|
Comm., Public & Agri. |
49 |
48 |
42 |
38 |
35 |
29 |
20 |
21 |
25 |
24 |
22 |
|
Combustion in
Industry |
|
|
|
|
|
|
|
|
|
|
|
|
Iron & Steel Combustion |
409 |
395 |
383 |
380 |
392 |
401 |
420 |
428 |
418 |
398 |
357 |
|
Processes in Industry |
56 |
47 |
44 |
39 |
46 |
48 |
45 |
44 |
44 |
41 |
43 |
|
Glass Production |
31 |
30 |
31 |
31 |
31 |
35 |
22 |
20 |
15 |
12 |
10 |
|
Other Comb. in Industry |
145 |
204 |
233 |
215 |
202 |
189 |
161 |
146 |
114 |
103 |
74 |
|
Production
Processes |
122 |
119 |
122 |
126 |
127 |
126 |
130 |
127 |
125 |
106 |
113 |
|
Non livestock
agriculture |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Waste Treatment
& Disp. |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Total |
2150 |
2212 |
2123 |
1971 |
1800 |
1542 |
1486 |
1331 |
1295 |
1200 |
1083 |
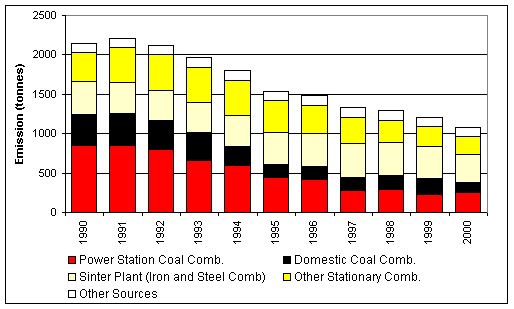
Figure 7.4 UK Emissions of Potassium
Accuracy of Emission Estimates Of Base Cations
Quantitative estimates of the uncertainties in emission inventories have been based on calculations made using a direct simulation technique, which corresponds to the IPCC Tier 2 approach recommended for greenhouse gases and also the methodology proposed in draft guidance produced by the UN ECE Taskforce on Emission Inventories. This work is described in detail by Passant (2002b). The estimates are shown in Table 7.5.
Table 7.5 Uncertainty of the Emission Inventories for Base Cations
|
Pollutant |
Estimated
Uncertainty % |
|
Calcium |
-50% to +100% |
|
Magnesium |
-40% to +80% |
|
Potassium |
-60% to +200% |
|
Sodium |
-40% to +100% |
Inventories for base cations have been significantly revised since the previous version of the NAEI, but many of the emission estimates are still subject to significant uncertainty. This is because they are based on emission estimates for PM10 (which are themselves highly uncertain), coupled with estimates of the chemical composition of the PM10 which add further uncertainty.