Acidifying Gases and Tropospheric Ozone Precursors
Introduction
The deposition of acidifying species can have adverse effects on buildings and vegetation, as well as acidifying streams and lakes and damaging the aquatic environment. Sulphur dioxide and nitrogen oxides from fuel combustion are major contributors to acidification (Review Group on Acid Rain-RGAR, 1997). Ammonia plays an important part in the long range transport of the acidifying pollutants by the formation of relatively stable particles of ammonium sulphate and ammonium nitrate. Although ammonia is a basic gas, deposition to soil surfaces and has an indirect effect on acidification. The biological transformation of NH4+ to NO3- in soils (nitrification) and plant uptake both release acidity into the soil contributing to acidification. NH3 deposition can also give rise to eutrophication- where nutrient enrichment gives rise to changes in ecosystems.
Tropospheric, or ground level, ozone occurs naturally and there are no significant ozone emissions from anthropogenic activities. Atmospheric levels can be increased in-situ by the photochemical reaction of precursor pollutants such as carbon monoxide, nitrogen oxides and volatile organic compounds. Specific NMVOC compounds and groups of compounds play a key role in ozone formation. Ozone episodes in which concentrations rise substantially above background levels occur in summer months when there are long periods of bright sunshine, temperatures above 20o C and light winds. Ozone can affect human health and can damage plants and crops. The total 2000 UK emissions of acidifying gases and ozone precursors are summarised in Table 5.1.
Table 5.1 Total UK Emissions of Acidifying and Ozone Precursors
|
Pollutant |
Total 2000
emission (kt) |
|
|
Nitrogen oxides |
1512 |
|
|
Sulphur dioxide |
1165 |
|
|
Hydrogen chloride |
84 |
|
|
Non-methane volatile organic compounds (NMVOC) |
1676 |
|
|
Ammonia |
319 |
|
|
Hydrogen fluoride |
3.8 |
|
The UK is committed to reducing acidifying gas and ozone precursor emissions and is a party to several protocols under the UN/ECE’s Convention on Long-Range Transboundary Air Pollution.
Under the Second Sulphur Protocol, the UK must reduce its total SO2 emissions by 50% by 2000, 70% by 2005 and 80% by 2010 (all from a 1980 baseline). The UK is well on track to meet these targets, and by the end of 2000 had achieved a 76% reduction from 1980 baseline levels, 26% ahead of the UN/ECE target level for the year 2000.
The VOC Protocol requires the UK to achieve a 30% reduction of anthropogenic VOC emissions by 1999 from a 1988 baseline. The emission estimates given in the 1999 version of the emissions inventory indicated that this was achieved. Emissions excluding those from forests fell from 2475 ktonnes in 1988 to 1577 ktonnes in 1999 - a reduction of 36%. This reduction was achieved largely as a result of emission controls for road vehicles and industrial processes, introduced by European Directives and the Environmental Protection Act 1990 respectively. Other factors also had an impact:
· prohibition of the burning of crop residues in England and Wales since 1993, except in limited cases of exemption.
· a decline in the use of coal as a fuel by electricity generators, industry and domestic users in favour of gas;
· a decline in the use of petrol as a fuel for cars in favour of DERV;
· improvements in technology introduced for economic reasons, or in response to health & safety legislation (e.g. the introduction of more efficient dry cleaning machines with lower emission levels);
· measures introduced either voluntarily, or in response to pressure from end-users for improved environmental or health and safety performance (e.g. the formulations of many consumer products have been changed, resulting in lower levels of solvent in those products and therefore lower emissions of VOC during their use).
The NOx Protocol required that the total emissions of NOx in 1994 should be no higher than they were in 1987; UK emissions were 17% lower in 1994 than in 1987 and have fallen substantially since 1994.
In 1996, the UN/ECE started negotiating a new multieffect, multipollutant protocol on nitrogen oxides and related substances. This was aimed at addressing photochemical pollution, acidification and eutrophication. The Protocol to Abate Acidification, Eutrophication and Ground-level Ozone was adopted in Gothenburg in December 2000, where it was signed by the UK. The multipollutant protocol incorporates several measures to facilitate the reduction of emissions:-
· Emission ceilings are specified for sulphur, nitrogen oxides, NH3 and NMVOCs. These are summarised in the following table.
Table 5.2 Emissions Ceilings for 2010 (ktonnes)
|
Country |
Sulphur (as SO2) |
NOx (as NO2) |
NH3 |
VOC |
|
Armenia |
73 |
46 |
25 |
81 |
|
Austria |
39 |
107 |
66 |
159 |
|
Belarus |
480 |
255 |
158 |
309 |
|
Belgium |
106 |
181 |
74 |
144 |
|
Bulgaria |
856 |
266 |
108 |
185 |
|
Croatia |
70 |
87 |
30 |
90 |
|
Czech Rep. |
283 |
286 |
101 |
220 |
|
Denmark |
55 |
127 |
69 |
85 |
|
Finland |
116 |
170 |
31 |
130 |
|
France |
400 |
860 |
780 |
1100 |
|
Germany |
550 |
1081 |
550 |
995 |
|
Greece |
546 |
344 |
73 |
261 |
|
Hungary |
550 |
198 |
90 |
137 |
|
Ireland |
42 |
65 |
116 |
55 |
|
Italy |
500 |
1000 |
419 |
1159 |
|
Latvia |
107 |
84 |
44 |
136 |
|
Liechtenstein |
0.11 |
0.37 |
0.15 |
0.86 |
|
Lithuania |
145 |
110 |
84 |
92 |
|
Luxembourg |
4 |
11 |
7 |
9 |
|
Netherlands |
50 |
266 |
128 |
191 |
|
Norway |
22 |
156 |
23 |
195 |
|
Poland |
1397 |
879 |
468 |
800 |
|
Portugal |
170 |
260 |
108 |
202 |
|
Rep. of Moldova |
135 |
90 |
42 |
100 |
|
Romania |
918 |
437 |
210 |
523 |
|
Slovakia |
110 |
130 |
39 |
140 |
|
Slovenia |
27 |
45 |
20 |
40 |
|
Spain |
774 |
847 |
353 |
669 |
|
Sweden |
67 |
148 |
57 |
241 |
|
Switzerland |
26 |
79 |
63 |
144 |
|
Ukraine |
1457 |
1222 |
592 |
797 |
|
United Kingdom |
625 |
1181 |
297 |
1200 |
· The protocol gives emission limits for sulphur, nitrogen oxides and NMVOCs from stationary sources.
· The protocol indicates limits for CO, hydrocarbons, nitrogen oxides and particulates from new mobile sources
· Environmental specifications for petrol and diesel fuels are given.
· Several measures to reduce NH3 emissions from the agriculture sector are also given.
The Gothenburg protocol forms a part of the Convention on Long-range Transboundary Air Pollution. More detailed information on both of the Gothenburg protocol and the Convention on Long-range Transboundary Air Pollution may be found at the UN/ECE web site:- http://www.unece.org/env/lrtap/
Within the EU, the
National Emission Ceilings Directive sets emission ceilings for 2010 for each
Member State for the same 4 pollutants as in the Gothenburg Protocol. A number
of Member States reduced their ceilings somewhat below the levels included in
the Protocol. The UK reduced its SO2 ceiling to 585 ktonnes and its
NOx ceiling to 1167 ktonnes.
Sulphur dioxide has long been recognised as a pollutant because of its role, along with particulate matter, in forming winter-time smogs. Estimates of sulphur dioxide emissions have been produced since the inception of the NAEI. Fuel combustion accounts for more than 95% of UK SO2 emissions with the sulphur arising from the fuel itself. The SO2 emission can be calculated from knowledge of the sulphur content of the fuel and from information on the amount of sulphur retained in the ash. Published fuel consumption data (DTI, 1998), published sulphur contents of liquid fuels (Institute of Petroleum, 1996) and data from coal producers regarding sulphur contents of coals enable reliable estimates to be produced.
The main sources of NOx in the UK are also combustion processes. However, such emissions are complex since the nitrogen can be derived from both the fuel and atmospheric nitrogen. The emission is dependent on the conditions of combustion, in particular temperature and excess air ratio, which can vary considerably. Thus combustion conditions, load and even state of maintenance are important. The estimation of NOx emissions is often based on relatively few measurements and, in view of the possible variation in emissions from apparently similar combustion plant, there is greater uncertainty in the estimates than for SO2 .
Within the UK, the implementation of the EC’s Large Combustion Plant Directive and other associated policy measures has led to substantial reductions in acidifying pollutants from power plants and industrial sources. Emissions of NOx from road traffic peaked in 1989 but by 2000 had substantially declined.
The inventories for SO2, NOx , HCl, NMVOC, NH3 and HF are discussed in the following sections. Full details of the methodologies used to compile the inventories, changes to the methodology since the 1998 inventory and detailed time series for these pollutants are presented in the Appendices (see the NAEI website: http://www.naei.org.uk).
NOx Emission estimates
Since 1970 there has been a reduction in total NOx emissions of 39%, however this decrease in emissions has not been constant (Figure 5.1). Up to 1984 the NOx emission profile was relatively flat with small peaks in 1973 and 1979, as seen previously for CO2, which were due largely to the cold winters in those years. However, from 1984, emissions rose markedly as a result of the growth in road traffic reaching a peak in 1989 (Table 5.3). Since 1989, total emissions have declined by 46% as a result of a 54% reduction from power stations and 53% decrease from road transport.
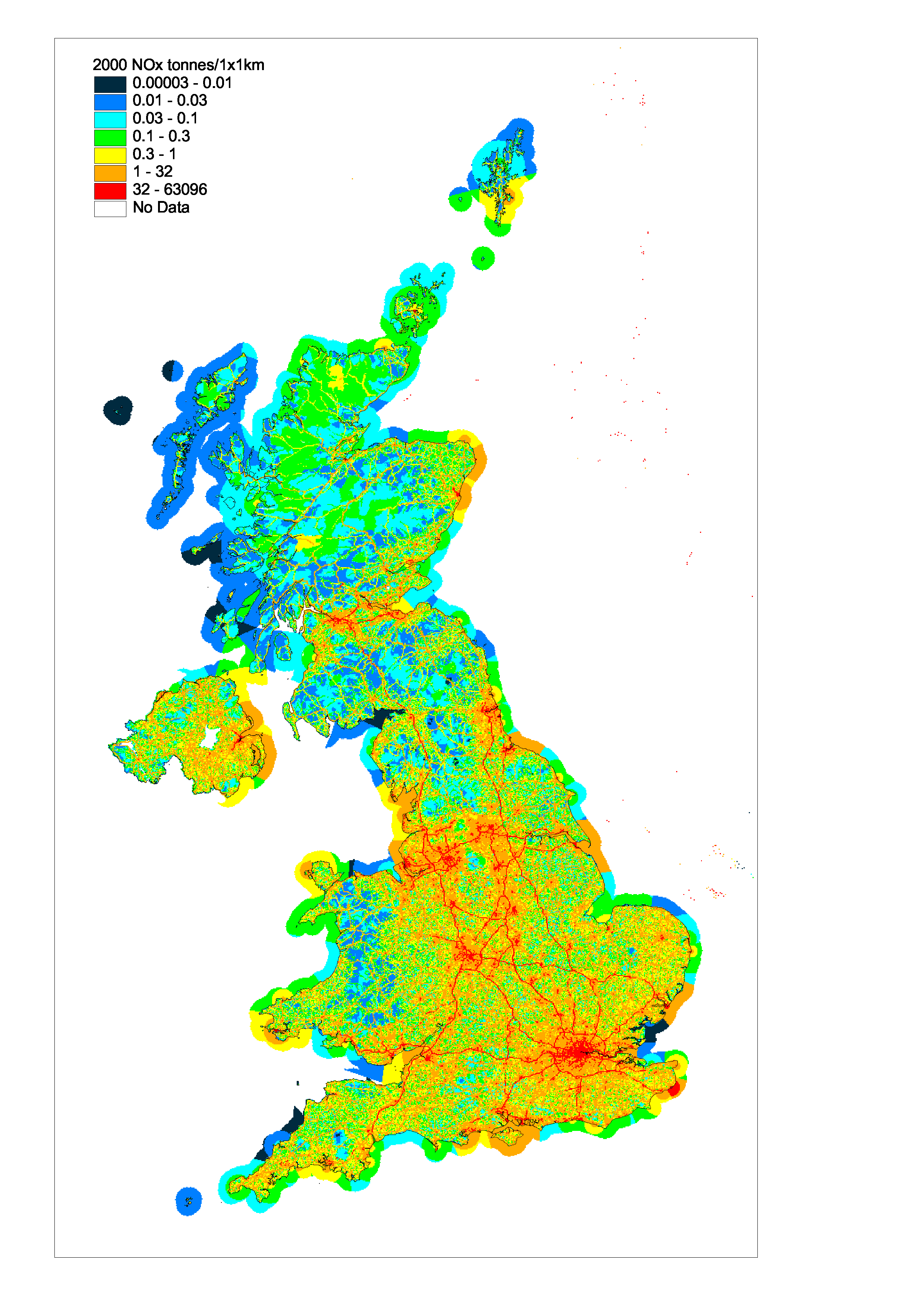
The spatially disaggregated emission inventory for the UK based on a 1x1 km grid is shown in Figure 5.2 and data files are also available from the NAEI’s internet site http://www.naei.org.uk A large fraction (the order of 30%) of the total emission is concentrated in a few grid squares which contain point sources. For NOx road transport dominates with approximately one third of the UK NOx emission deriving from major sections of road. Vehicles travelling at high speeds contribute most. As a result the major route-ways (e.g. Motorways and primary routes) are clearly defined on the map. Conurbations and city centres show high emissions resulting from large volumes of road transport, residential and commercial combustion. A combination of high national shipping emission and relatively few large ports result in significant localised emissions from shipping in port areas.
Table 5.3 UK Emissions of Nitrogen Oxides by UN/ECE1 Source Category and Fuel (kt)
|
|
1970 |
1980 |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2000% |
|
BY UN/ECE CATEGORY2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comb. in Energy
Prod. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
812 |
861 |
781 |
683 |
671 |
567 |
527 |
495 |
449 |
372 |
364 |
338 |
358 |
24% |
|
Petroleum Refining Plants |
43 |
42 |
40 |
41 |
40 |
37 |
36 |
35 |
35 |
33 |
39 |
32 |
28 |
2% |
|
Other Comb. & Trans. |
56 |
42 |
61 |
62 |
63 |
65 |
75 |
47 |
47 |
47 |
50 |
53 |
52 |
3% |
|
Comb. in
Comm/Inst/Res |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Residential Plant |
62 |
64 |
64 |
71 |
69 |
72 |
69 |
66 |
75 |
69 |
71 |
71 |
72 |
5% |
|
Comm/Pub/Agri Comb. |
74 |
46 |
38 |
41 |
40 |
38 |
38 |
38 |
39 |
36 |
34 |
34 |
32 |
2% |
|
Combustion in
Industry |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iron & Steel Comb. |
86 |
28 |
28 |
27 |
27 |
28 |
32 |
31 |
31 |
31 |
28 |
30 |
27 |
2% |
|
Other Ind. Comb. |
325 |
246 |
197 |
187 |
183 |
180 |
188 |
173 |
166 |
169 |
162 |
147 |
137 |
9% |
|
Production
Processes |
13 |
14 |
11 |
9 |
9 |
8 |
7 |
4 |
4 |
4 |
4 |
4 |
4 |
0% |
|
Extr./Distrib. of
Fossil Fuels |
0 |
0 |
1 |
1 |
1 |
0 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
0% |
|
Road Transport |
771 |
990 |
1305 |
1274 |
1224 |
1147 |
1084 |
997 |
956 |
881 |
788 |
716 |
629 |
42% |
|
Other Trans/Mach |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Off-Road Sources |
121 |
116 |
131 |
133 |
126 |
122 |
111 |
110 |
114 |
110 |
99 |
94 |
91 |
6% |
|
Other3 |
120 |
104 |
89 |
92 |
92 |
90 |
88 |
85 |
89 |
87 |
88 |
81 |
78 |
5% |
|
Waste |
6 |
12 |
9 |
8 |
8 |
6 |
8 |
8 |
7 |
4 |
4 |
3 |
3 |
0% |
|
Land Use Change |
10 |
15 |
9 |
8 |
6 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0% |
|
By FUEL TYPE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Solid |
888 |
867 |
776 |
691 |
675 |
551 |
505 |
475 |
420 |
345 |
331 |
302 |
311 |
21% |
|
Petroleum |
1388 |
1422 |
1662 |
1628 |
1564 |
1488 |
1401 |
1289 |
1253 |
1148 |
1041 |
947 |
846 |
56% |
|
Gas |
98 |
171 |
216 |
224 |
234 |
244 |
267 |
243 |
266 |
270 |
282 |
291 |
299 |
20% |
|
Non-Fuel |
125 |
120 |
110 |
93 |
86 |
77 |
90 |
82 |
75 |
80 |
77 |
64 |
57 |
4% |
|
TOTAL |
2499 |
2580 |
2763 |
2637 |
2558 |
2361 |
2263 |
2088 |
2014 |
1844 |
1732 |
1604 |
1512 |
100% |
1 UK emissions reported in IPCC format (Salway, 2002) differ slightly
due to the different source categories
used.
2 See Annex 1 for definition of UN/ECE Categories
3 Including railways,
shipping, naval vessels, military aircraft
Figure 5.1 Emission Profile NOx Emissions
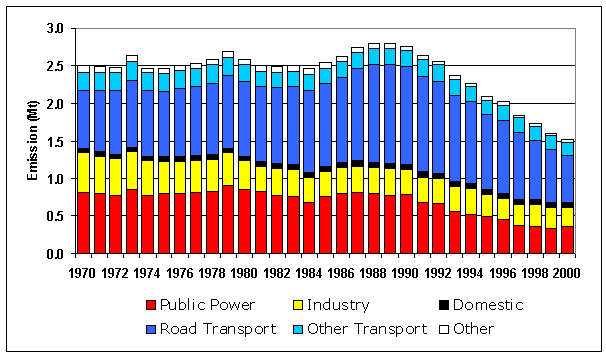
Figure 5.2 Spatially Disaggregated UK Emissions of NOx
Transport
The major source of NOx emissions in the UK is the transport sector with road vehicles and off-road vehicles contributing 42% and 6%, respectively, to the total emission. Road emissions rose steadily between 1970 and 1989 reflecting the overall growth in road traffic in the UK. During this period emissions from total petrol consumption, predominantly cars, rose by 102% compared to the 1970 level and emissions from DERV consumption rose by 40%. Figure 5.3 clearly shows the growth in the vehicle fleet and vehicle mileage during this period. Since 1989 there has been a steady decline in emissions due to the introduction of catalytic converters on cars and stricter regulations on truck emissions.
Figure 5.3 Emissions of NOx from Road Transport by Vehicle Type
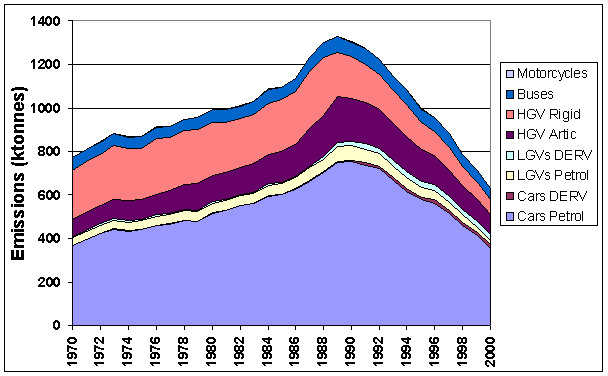
Figure 5.4 shows the average NOx emissions per vehicle kilometre for different vehicle types (NOTE: two different Y scales are used). Various emission regulations on new petrol cars which have come into effect in stages since 1976 have led to the gradual reduction in emission rates from petrol cars. The more rapid decline in emissions from 1992 is due to the penetration of cars fitted with three-way catalysts. Limits on emissions from diesel cars and Light Goods Vehicles (LGVs) did not first come into effect until 1993/94. Overall emissions per kilometre from Heavy Goods Vehicles (HGVs) showed a small rise from 1970-1987 due to the increasing usage of larger HGVs for freight movement. Limits on emissions from HGVs first came into effect in 1988 leading to a gradual reduction in emission rates as new HGVs penetrated the fleet, accelerated by tighter limits on emissions from new HGVs in 1993/94.
Figures 5.4 NOx Emissions per Vehicle km by Vehicle Type.
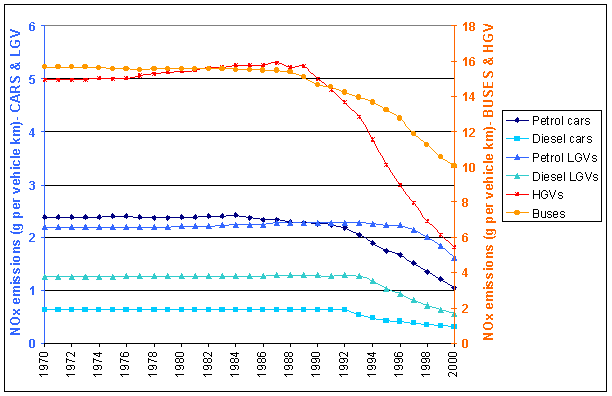
Figure 5.5 shows emissions per passenger km and by tonne km of freight. Technological improvements to HGVs give rise to approximately half the emissions per tonne of freight moved in 1998 compared with 1970. Emissions per passenger km from cars, vans and taxies have significantly decreased since 1970 due mainly to the introduction of catalytic convertors in 1992 now penetrating the car fleet. Per passenger km emissions from buses and coaches have increased from 1970 to 1993. This was due to the gradual decrease in occupancy rate of buses and their under utilisation over this period. Since 1993, this rise in per passenger km emissions has been halted by the penetration of buses meeting tighter emission standards into the fleet.
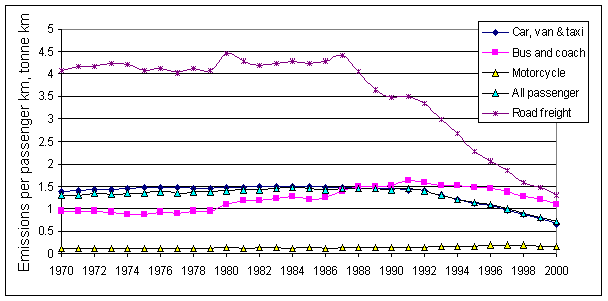
Figure 5.5 Emissions of NOx
(grams) by Mode for Passenger and Freight Transport.
Other transport and machinery contribute a further 11% to total UK NOx emissions. Of these only those from civil aircraft have grown steadily over the period 1970 to 2000 (take-off and landing cycles up to an altitude of 1000 m only are considered here in accordance with UN/ECE guidelines). However, these emissions contribute only a small percentage of the total emission.
Power Generation
Emissions from power stations have declined over the period 1970-2000 by 56%. Emissions in the seventies were fairly constant from year to year, with peaks in severe winters. Since 1979 emissions have declined with a significant decrease at the time of the miners strike in 1984. Prior to 1989 this decline was due to the increased use of nuclear power and an increase in the average efficiency of the thermal power stations. Since 1988 the electricity generators have adopted a programme of progressively fitting low NOx burners to their 500 MWe coal fired units. More recently the increased use of nuclear generation and the introduction of CCGT plant burning natural gas (See Section 2.2.2) have further reduced NOx emissions. The emissions from the low NOx turbines used are much lower than those of pulverised coal fired plant even when low NOx burners are fitted. Given that these trends continue, power station emissions are expected to fall further.
Industry
The emissions from industrial combustion have declined by 60% since 1970 and they currently contribute 11% to total UK emissions. This is due to the decline in coal use in favour of gas and electricity.
SO2 Emission EStimates
Since 1970 there has been a substantial overall reduction of more than 80% in SO2 emissions (Figure 5.6). The emission profile exhibits a steady decline between 1970 and 2000 with the exception of small peaks in 1973 and 1979 corresponding to the harsh winters in those years and a short period at the end of the 1980s when emissions were relatively constant from year to year. It is also evident that there is little decrease between total SO2 emissions in 1997 and 1998. This occurs because the large reductions in emissions from the power generation sector are not as substantial between 1997 and 1998. However the trend resumes between 1998 and 2000.
Table 5.3 shows emissions broken down by fuel categories. The two main contributors are solid fuel and petroleum products. Emissions from solid fuel use have declined by 75% since 1970 and those from petroleum by 93%. The most important factors in the fall in emissions from petroleum use are the decline in fuel oil use and the reduction in the sulphur content of gas oil and DERV. The reduction in the sulphur content of gas oil is particularly significant in sectors such as domestic heating, commercial heating and off-road sources where gas oil is used extensively. The sulphur content of DERV has steadily reduced across recent years, giving rise to a significant reduction in SO2 emissions. SO2 emissions from DERV in the early 1990’s were relatively constant, however between 1994 and 2000 there has been a 97% reduction in emissions.
Figure 5.6 Emissions profile for SO2
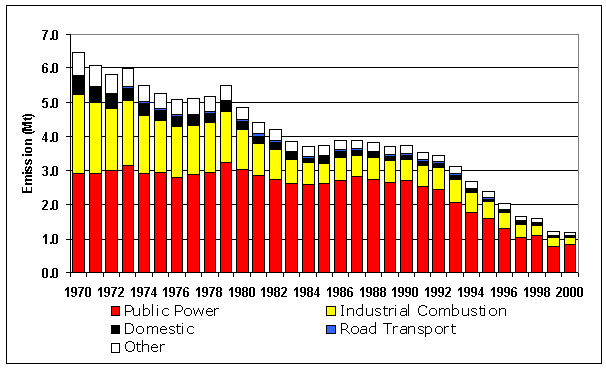
Table 5.4 UK Emissions of Sulphur Dioxide by UN/ECE1 Source Category and Fuel (kt)
|
|
1970 |
1980 |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2000% |
|
BY UN/ECE CATEGORY2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comb. in Energy
Prod. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
2913 |
3007 |
2723 |
2535 |
2434 |
2083 |
1762 |
1591 |
1320 |
1025 |
1072 |
776 |
826 |
71% |
|
Petroleum Refining Plants |
242 |
262 |
153 |
161 |
146 |
148 |
135 |
142 |
144 |
134 |
98 |
93 |
72 |
6% |
|
Other Comb. & Trans. |
231 |
25 |
8 |
8 |
6 |
5 |
4 |
3 |
3 |
6 |
7 |
9 |
8 |
1% |
|
Comb. in
Comm/Inst/Res |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Residential Plant |
522 |
226 |
108 |
115 |
103 |
113 |
92 |
67 |
71 |
63 |
53 |
52 |
44 |
4% |
|
Comm/Pub/Agri Comb. |
451 |
218 |
93 |
88 |
90 |
95 |
81 |
60 |
57 |
47 |
34 |
27 |
17 |
1% |
|
Combustion in Industry |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iron & Steel Comb. |
378 |
105 |
54 |
52 |
56 |
65 |
58 |
53 |
47 |
46 |
37 |
32 |
23 |
2% |
|
Other Ind. Comb. |
1470 |
815 |
390 |
400 |
454 |
441 |
380 |
298 |
248 |
225 |
178 |
131 |
102 |
9% |
|
Production
Processes |
115 |
88 |
61 |
57 |
52 |
49 |
46 |
45 |
44 |
41 |
41 |
37 |
31 |
3% |
|
Extr./Distrib. of
Fossil Fuels |
5 |
5 |
16 |
6 |
7 |
5 |
6 |
6 |
7 |
6 |
6 |
1 |
1 |
0% |
|
Road Transport |
44 |
42 |
63 |
58 |
62 |
59 |
63 |
51 |
37 |
27 |
23 |
14 |
6 |
1% |
|
Other Trans/Mach3 |
93 |
61 |
47 |
50 |
49 |
48 |
46 |
44 |
44 |
42 |
38 |
34 |
32 |
3% |
|
Waste |
4 |
5 |
5 |
5 |
4 |
3 |
3 |
3 |
2 |
1 |
2 |
4 |
3 |
0% |
|
By FUEL TYPE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Solid |
3684 |
3151 |
2780 |
2679 |
2584 |
2173 |
1847 |
1645 |
1363 |
1158 |
1180 |
877 |
907 |
78% |
|
Petroleum |
2545 |
1548 |
811 |
746 |
746 |
844 |
720 |
604 |
558 |
395 |
294 |
229 |
175 |
15% |
|
Gas |
60 |
11 |
10 |
9 |
39 |
12 |
12 |
11 |
12 |
19 |
19 |
19 |
14 |
1% |
|
Non-Fuel |
178 |
149 |
120 |
99 |
93 |
86 |
97 |
102 |
92 |
93 |
95 |
84 |
70 |
6% |
|
TOTAL |
6468 |
4859 |
3721 |
3534 |
3462 |
3115 |
2676 |
2363 |
2025 |
1665 |
1588 |
1210 |
1165 |
100% |
1 UK emissions reported in IPCC format (Salway, 2002) differ slightly
due to the different source categories
used.
2 See Annex 1 for definition of UN/ECE Categories
3 Including railways,
shipping, naval vessels, military aircraft and off-road sources
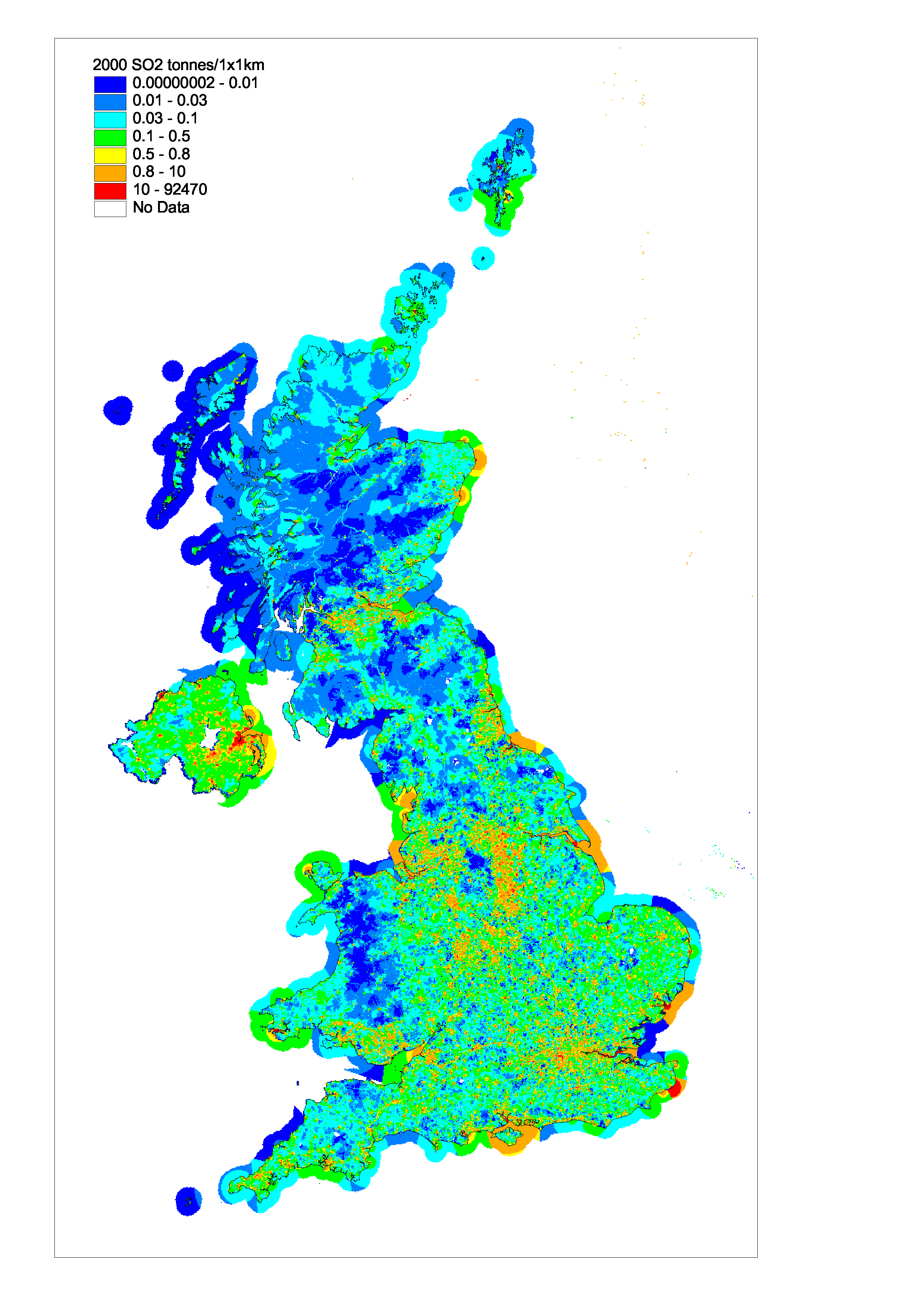
The geographical distribution of SO2 emissions is shown in Figure 5.7. A large fraction (the order of 80%) of the SO2 emissions are concentrated into relatively few 1x1 km grid squares containing the major point sources such as refineries and power stations and large industrial plant. The resulting map highlights the main conurbations and some major roads. High emissions in Plymouth and Newport result from a combination of shipping and industry. London and Birmingham, which are covered by Smoke Control Areas, show relatively low SO2 emission levels. High emission densities are noted in Belfast where there is substantial consumption of solid fuels in the domestic sector for heating etc.
Figure 5.7 Spatially Disaggregated UK Emissions of SO2
Power Generation
The largest contribution to SO2 emissions is from power stations which accounts for 71% of the total in 2000. Historically coal combustion has been the most important source- the sulphur content of the coal being directly proportional to the emission estimate. Since 1970 there has been a gradual decline in power station emissions of around 72%. This reflects the changes in fuel mix and the types of power plant which have taken place during the period. From 1970 to 1990 the reduction was due to a gradual increase in the use of nuclear plant and improvements in efficiency (See Section 2.2.2). Since 1990, this decline has accelerated because of the increase in the proportion of electricity generated in nuclear plant and the use of Combined Cycle Gas Turbine (CCGT) stations and other gas fired plant. CCGTs are more efficient than conventional coal and oil stations and have negligible SO2 emissions. It is expected that these reductions will continue in the near future as more CCGT stations are built. Most recently the flue gas desulphurisation plants, constructed at Drax and Ratcliffe power stations have had a significant effect on emissions.
Industry
Emissions of SO2 from industry result from the combustion of coal and oil, some refinery processes and the production of sulphuric acid and other chemicals. Between 1970 and 2000 industrial emissions from combustion sources have fallen by 93% though most of the fall took place between 1970-1985 reflecting the decline in the energy intensive iron and steel industry and other heavy industries. There has been also been a decline in the use of coal and oil in favour of natural gas.
Transport
Transport emissions account for just 1% of the total SO2 emissions. Between 1970 and the early 1990s, road transport emissions grew with the increase in road vehicles, however more recently emissions have declined with the reduction in the sulphur content of DERV. Similarly the reduction in sulphur content of gas oil is reflected in the emissions from off-road vehicles.
Other
Emissions from the remaining categories are low compared with those discussed above. Emissions from domestic and other commercial/institutional sectors have declined substantially during the period 1970-2000, reflecting the major changes in fuel mix from oil and coal to gas. The decrease in emissions from waste reflects the closure of a number of old incinerators due to the introduction of new emission standards and their replacement with modern equipment.
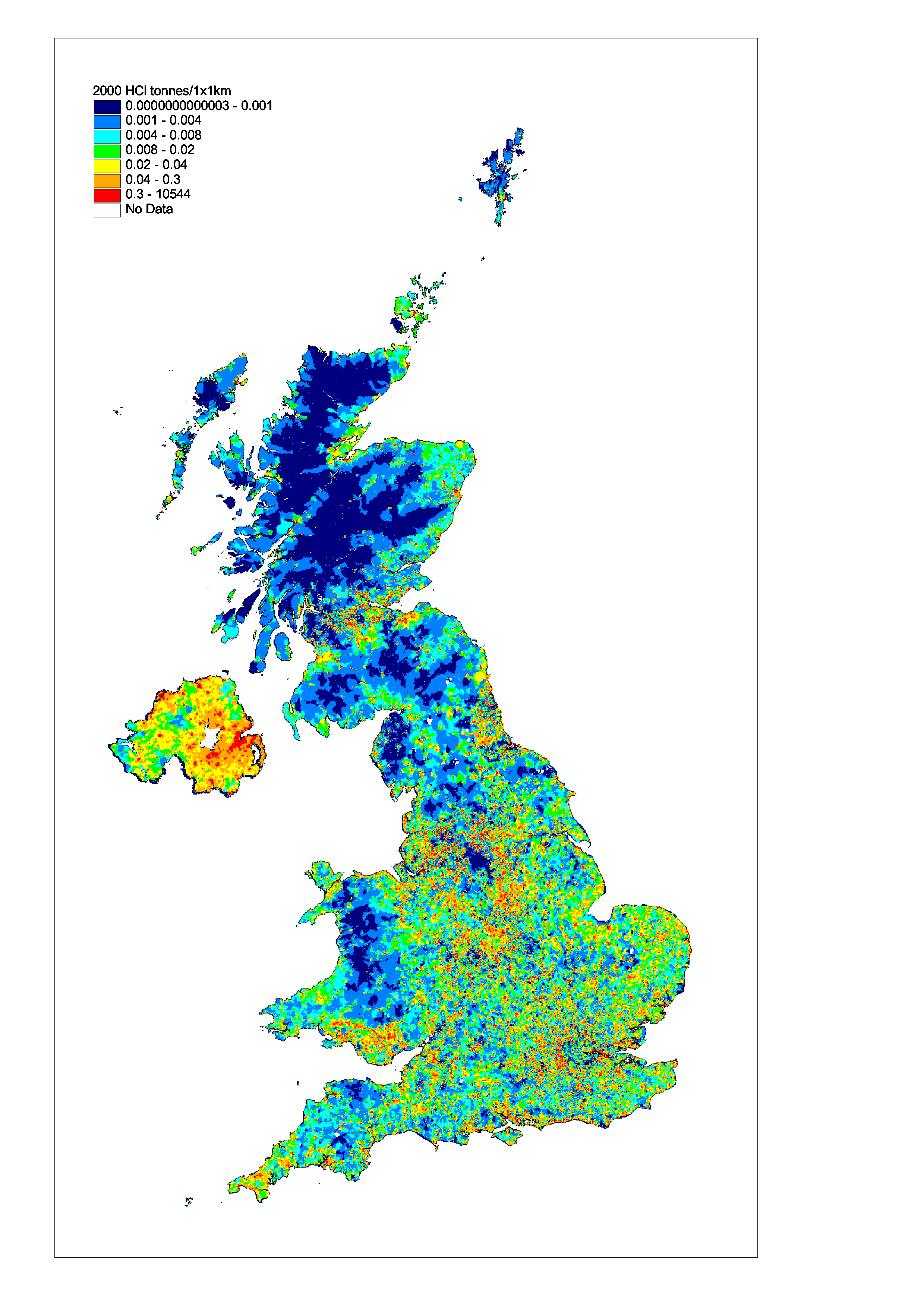
Hydrogen chloride emission estimates
HCl is an acidic gas primarily released to air from combustion of fuels which contain trace amounts of chloride. This results in the emissions of HCl being dominated by the combustion of solid fuel.
Table 5.5 and Figure 5.8 summarise the UK emissions of hydrogen chloride. Emissions have fallen by 75% since 1970. The main source of these emissions is coal combustion so the fall is a result of the decline in coal use and also the installation of flue gas desulphurisation at Drax and Ratcliffe since 1993. The other significant source of hydrogen chloride is waste incineration. Here the commissioning of new incinerators and the closure or upgrading of old plant has resulted in a large decrease for all years since 1996.
Table 5.5 UK Emissions of Hydrogen Chloride by UN/ECE Source Category and Fuel (kt)
|
|
1970 |
1980 |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2000% |
|
BY UN/ECE CATEGORY1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comb. in Energy
Prod. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
222.3 |
257.8 |
239.4 |
237.9 |
223.9 |
186.0 |
170.5 |
133.2 |
109.4 |
74.5 |
76.7 |
82.2 |
72.4 |
86% |
|
Petroleum Refining Plants |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0% |
|
Other Comb. & Trans. |
4.5 |
1.6 |
0.3 |
0.3 |
0.2 |
0.1 |
0.1 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0% |
|
Comb. in
Comm/Inst/Res |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Residential Plant |
47.3 |
21.0 |
10.0 |
11.2 |
9.8 |
10.9 |
9.2 |
6.3 |
6.4 |
6.1 |
5.6 |
5.9 |
4.5 |
5% |
|
Comm/Pub/Agri Comb. |
9.9 |
4.3 |
3.6 |
3.6 |
3.0 |
2.3 |
2.4 |
2.0 |
2.1 |
1.5 |
1.0 |
0.9 |
0.7 |
1% |
|
Combustion in
Industry |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iron & Steel Comb. |
1.8 |
0.4 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0% |
|
Other Ind. Comb. |
40.9 |
14.4 |
15.1 |
16.3 |
18.5 |
17.1 |
16.1 |
15.1 |
13.0 |
11.9 |
9.4 |
8.8 |
6.4 |
8% |
|
Production
Processes |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.3 |
0.3 |
0.2 |
0.2 |
0.2 |
0.2 |
0% |
|
Road Transport |
0.4 |
0.4 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.0 |
0.0 |
0.0 |
0.0 |
0% |
|
Other Trans/Mach2 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0% |
|
Waste |
10.6 |
10.6 |
8.0 |
7.9 |
7.6 |
3.2 |
4.4 |
4.5 |
4.1 |
0.0 |
0.1 |
0.1 |
0.1 |
0% |
|
By FUEL TYPE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Solid |
325.0 |
298.7 |
264.8 |
265.6 |
251.3 |
213.6 |
191.0 |
149.0 |
123.3 |
92.2 |
90.8 |
96.2 |
82.7 |
98% |
|
Petroleum |
0.7 |
0.6 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.0 |
0% |
|
Gas |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0% |
|
Non-Fuel |
12.2 |
11.5 |
11.9 |
11.8 |
11.8 |
6.2 |
11.8 |
12.3 |
11.9 |
2.1 |
2.2 |
1.9 |
1.5 |
2% |
|
TOTAL |
338 |
311 |
277 |
278 |
263 |
220 |
203 |
161 |
135 |
94 |
93 |
98 |
84 |
100% |
1 See Annex 1 for definition of UN/ECE Categories
2 Including railways,
shipping, naval vessels, military aircraft and off-road sources
Figure 5.8 Emissions Profile for Hydrogen Chloride
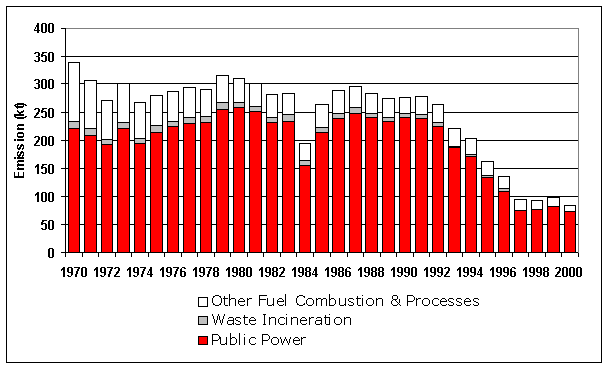
Figure 5.9, Spatially Disaggregated UK Emissions of HCl
Non-methane VOlatile organic Compounds
NMVOCs are a organic compounds which may differ widely in their chemical composition. These organic compounds are often grouped under the NMVOC label as the majority display similar behaviour in the atmosphere. NMVOCs are emitted to air as combustion products, as vapour arising from handling or use of petroleum distillates, solvents or chemicals, and from numerous other sources.
Interest in NMVOC emissions has grown as their role in the photochemical production of ozone has been appreciated. The diversity of processes which emit NMVOCs is huge, covering not only many branches of industry, but also transport, agriculture and domestic sources.
The NMVOC inventory is summarised in Table 5.6. Only 31% of the NMVOC emissions arise from combustion sources (unlike SO2 and NOx where the contribution from combustion sources is much higher). Of these emissions from combustion sources, it is the transport sector which dominates. Other major sources of NMVOC emissions are the use of solvents and industrial processes. Natural emissions of NMVOCs are included here as per UN/ECE guidelines (see Section 5.5.3). The NMVOC emission profile, Figure 5.10, shows a small overall increase in emissions between 1970 and 1989 with minor peaks in 1973 and 1979, followed by a steady reduction in emissions during the 1990s. The latter is largely a reflection of the increasingly stringent emission limits across a range of sectors.
Figure 5.10 NMVOC Emissions Profile
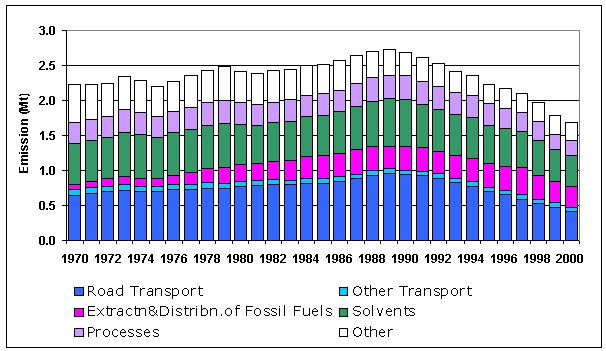
Table 5.6 UK Emissions of NMVOCs by UN/ECE1 Source Category and Fuel (kt)
|
|
1970 |
1980 |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2000% |
|
BY UN/ECE CATEGORY2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comb. in Energy
Prod. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
7 |
8 |
7 |
7 |
7 |
7 |
8 |
8 |
9 |
8 |
6 |
8 |
8 |
0% |
|
Petroleum Refining Plants |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
0% |
|
Other Comb. & Trans. |
2 |
2 |
2 |
2 |
3 |
3 |
3 |
1 |
1 |
1 |
1 |
1 |
1 |
0% |
|
Comb. in
Comm/Inst/Res |
296 |
131 |
67 |
70 |
65 |
64 |
52 |
40 |
44 |
40 |
42 |
45 |
36 |
2% |
|
Combustion in
Industry |
19 |
14 |
9 |
8 |
8 |
8 |
9 |
9 |
9 |
9 |
9 |
7 |
8 |
0% |
|
Production
Processes |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Petroleum Refining Plants |
107 |
99 |
100 |
102 |
102 |
98 |
98 |
87 |
74 |
66 |
59 |
46 |
45 |
3% |
|
Chemicals manufacture |
94 |
113 |
148 |
138 |
136 |
131 |
122 |
140 |
139 |
125 |
113 |
78 |
74 |
4% |
|
Food & Drink Manufacture |
74 |
82 |
73 |
74 |
74 |
75 |
75 |
76 |
78 |
79 |
80 |
80 |
78 |
5% |
|
Other processes |
16 |
13 |
18 |
17 |
17 |
17 |
18 |
17 |
16 |
13 |
10 |
10 |
10 |
1% |
|
Extr./Distrib. of
Fossil Fuels |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gas Leakage |
12 |
38 |
42 |
41 |
40 |
40 |
39 |
39 |
38 |
66 |
66 |
66 |
63 |
4% |
|
Offshore Oil&Gas |
5 |
98 |
144 |
149 |
138 |
140 |
165 |
161 |
173 |
173 |
151 |
147 |
150 |
9% |
|
Gasoline Distribution |
65 |
101 |
147 |
147 |
148 |
147 |
141 |
135 |
137 |
137 |
127 |
88 |
90 |
5% |
|
Solvent Use |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Industrial solvent use |
476 |
449 |
473 |
437 |
407 |
403 |
403 |
368 |
355 |
344 |
334 |
296 |
276 |
16% |
|
Domestic solvent use |
112 |
126 |
192 |
191 |
185 |
179 |
177 |
173 |
169 |
169 |
169 |
167 |
169 |
10% |
|
Road Transport |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Combustion |
523 |
601 |
697 |
679 |
647 |
600 |
562 |
514 |
478 |
434 |
389 |
351 |
303 |
18% |
|
Evaporation |
121 |
171 |
241 |
237 |
233 |
217 |
201 |
185 |
172 |
158 |
135 |
123 |
105 |
6% |
|
Other Trans/Mach3 |
78 |
72 |
66 |
67 |
67 |
66 |
64 |
63 |
64 |
63 |
62 |
61 |
61 |
4% |
|
Waste |
10 |
56 |
43 |
40 |
38 |
38 |
45 |
37 |
36 |
31 |
29 |
24 |
21 |
1% |
|
Land Use Change |
37 |
58 |
35 |
30 |
22 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0% |
|
Nature |
178 |
178 |
178 |
178 |
178 |
178 |
178 |
178 |
178 |
178 |
178 |
178 |
178 |
11% |
|
By FUEL TYPE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Solid |
300 |
132 |
66 |
68 |
63 |
61 |
49 |
38 |
39 |
35 |
34 |
37 |
27 |
2% |
|
Petroleum |
728 |
847 |
1006 |
985 |
949 |
885 |
830 |
764 |
716 |
656 |
588 |
537 |
470 |
28% |
|
Gas |
7 |
12 |
15 |
16 |
16 |
17 |
18 |
16 |
19 |
19 |
20 |
22 |
22 |
1% |
|
Non-Fuel |
1196 |
1419 |
1599 |
1547 |
1489 |
1448 |
1465 |
1415 |
1396 |
1386 |
1320 |
1183 |
1156 |
69% |
|
TOTAL |
2231 |
2410 |
2686 |
2616 |
2516 |
2411 |
2362 |
2232 |
2170 |
2097 |
1962 |
1778 |
1676 |
100% |
1 UK emissions reported in IPCC format (Salway, 2002) differ slightly
due to the different source categories
used.
2 See Annex 1 for definition of UN/ECE Categories
3 Including railways,
shipping, naval vessels, military aircraft and off-road sources
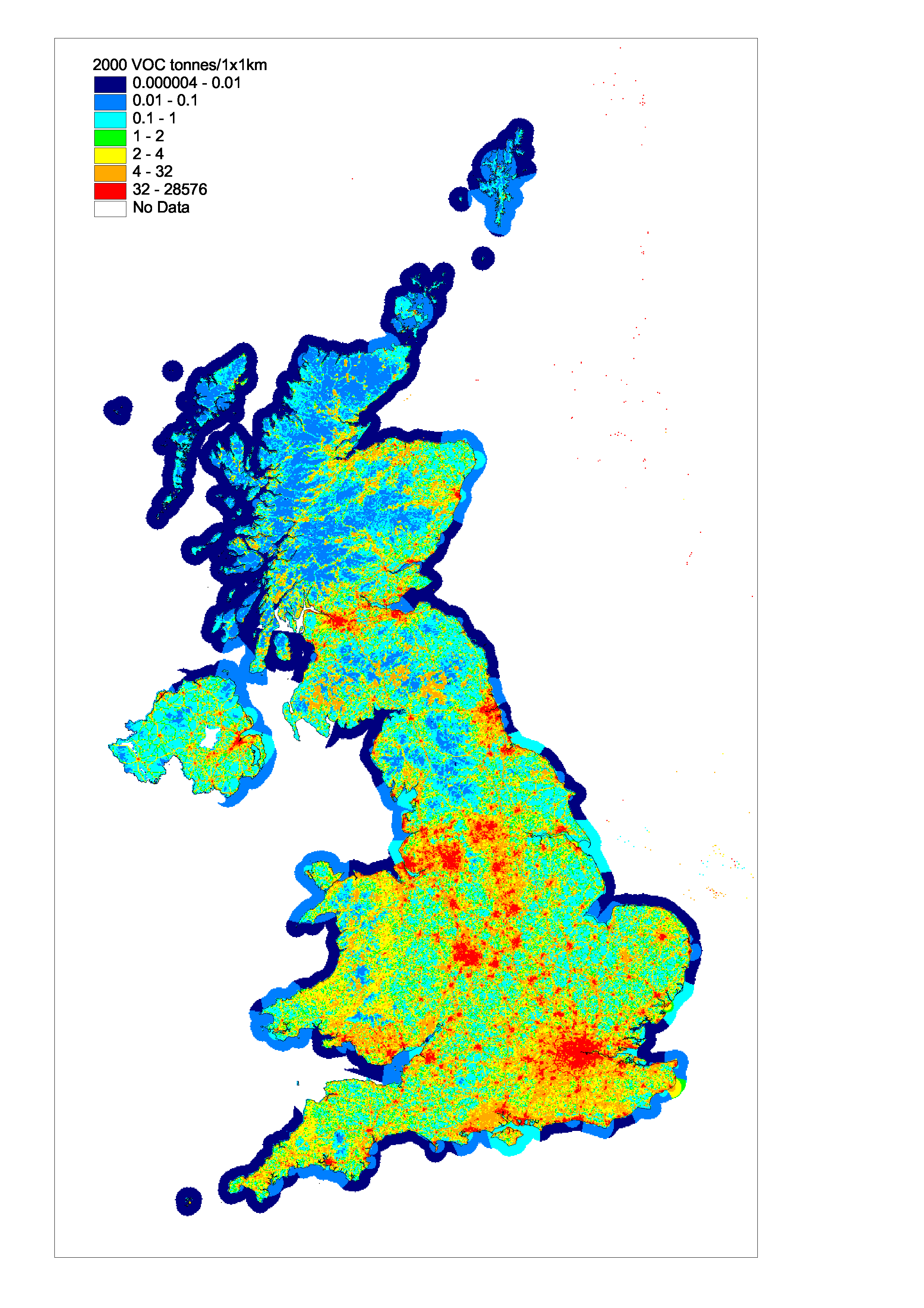
The spatial disaggregation of NMVOC emissions in the UK is shown in Figure 5.11. A large proportion of emissions are caused either as a result of the activities of people in and around their homes (e.g. domestic solvent use or domestic combustion), or by widespread industrial activities such as small-scale industrial coating processes, dry cleaning shops, and small bakeries, which are present in towns and cities throughout the UK. Consequently the resulting emissions map is well correlated with population density.
The NMVOC map includes a large number of point sources, including oil refineries, crude oil terminals, large combustion plant, chemicals manufacture, iron and steel processes, whisky manufacture, large bread bakeries, and industrial solvent using processes. The domestic sources are distributed using population density statistics, and the sources arising from other industrial processes are mapped using information on the size and locations of industrial installations.
Unlike the maps presented previously for SO2 and NOx, the VOC map has little major road definition except where the major roads go through rural areas. This reflects the fact that NMVOC emissions are dependent on vehicle speed and are higher on minor and urban major roads than on the high speed motorways and major roads.
Figure 5.11, Spatially Disaggregated UK Emissions of NMVOC
Solvent use and production processes
Solvent use and production processes are responsible for 53% and 25%, respectively, of the 2000 emission total. The estimates are derived either based on plant specific data provided by process operators or regulators or by use of appropriate emission factors combined with solvent consumption data or industrial production data. The NMVOC inventory has been subject to a continuous programme of review and improvement over the past decade (see Appendix 2 for a description of recent improvements), and estimates, especially those for the period 1988 – 2000, can be considered reasonably reliable.
The solvent use sector comprises both industrial and domestic applications, both being significant sources. Emissions from industrial solvent use reached their peak in 1973, then dipped to a low in 1982, before increasing again until 1989. Since 1989 emissions have fallen as a result of emission controls, technological changes, and reduced manufacturing output in some sectors. In comparison, domestic solvent emissions showed little trend until the mid 1980s when they increased sharply. Since 1991 however, emissions have fallen back by 12% due to a trend towards formulating products such as paints and aerosols with lower solvent contents.
The production processes sector includes emissions from the chemical industry, petroleum refining, and food and drink manufacture as well as minor sources such as iron and steel production and road construction. Emissions from the chemical industry grew steadily until 1989, since when tightening emission controls have led to a reduction in emissions of 51%. The emissions from the petroleum refining sector show little trend over the period from 1970 until 1994, but since then emission controls and, latterly, refinery closures have led to emissions falling by 53% since the 1994 figure.
Emissions from the food and drink industry comprised 5% (78.4 ktonnes) of the total NMVOC emission in 2000. The largest source is whisky maturation although bread baking, animal feed manufacture, fat and oil processing and barley malting are also important. Emissions from the sector peaked in 1980 before falling again. Since 1987, however, emissions have been increasing by an average of 1% per year.
Transport
Total transport emissions are currently responsible for 28% of NMVOC emissions of which 24% are a result of road transport. From 1970 the emission rose gradually with increasing car numbers, to a peak in 1989. Since then it has declined substantially owing to the increased use of catalytic converters and fuel switching from petrol to diesel cars. Emissions from the road transport sector for 2000 are now substantially lower than in 1970.
Other Sectors
Offshore oil and gas emissions have increased substantially since 1970 with the growth of the UK’s offshore industry and now constitute 9% of the 2000 emissions total. The most important sources of NMVOC emissions are tanker loading, flaring and fugitive emissions.
Emissions from gas leakage currently comprise around 4% of the total NMVOC emission. This estimate has been significantly revised upwards in light of new data. Even though the mass of mains gas being released has decreased (due to pipe replacement), there is an upward temporal trend of NMVOC emission. This is caused by the increasing NMVOC content of mains gas.
The evaporative losses from the distribution and marketing of petrol rose between 1970 and the early 1990s reflecting the growth in road transport. Since then they have decreased, partly as a result of fuel switching to diesel, and partly as a result of increasing usage of petrol vapour recovery systems to prevent emissions from petrol terminals and service stations. They currently account for 5% of national NMVOC emissions.
The contribution from domestic heating has fallen by more than a factor of 6 over the period 1970-2000 as the use of coal for domestic heating has declined. It now accounts for just 2% of the UK emission.
NMVOC emissions from waste treatment and disposal contribute 1% to national emissions. Data from the Environment Agency (2000) shows emissions from municipal waste incinerators to be very small.
NMVOCs, in particular isoprene and mono-terpenes, are emitted from several natural and agricultural sources- such as forests. The entries under Land Use Change and Nature in Table 5.6 represent emissions from forests and forestry, heathland, pastures and crops.
Speciation of NMVOCs
As mentioned previously, the term NMVOC covers a wide range of compounds and although a total NMVOC inventory is sufficient for some purposes, in other cases greater detail is required concerning the nature and concentration of individual compounds. For example, when assessing the photochemical production of ozone, individual species have different ozone creation potentials hence information is required on the concentration of individual species (QUARG, 1993). Table 5.7 shows the emissions of the 50 most important NMVOC species disaggregated as far as possible by source. A more detailed speciation of all NMVOCs estimated by the NAEI (currently over 600) is given on the NAEI website (http://www.naei.org.uk).
Table 5.7 The 50 Most Significant NMVOC Species in Terms of Mass Emission (ktonnes)
|
|
Stationary Combustion
(Energy Production) |
Stationary Combustion
(Commercial & Residn) |
Stationary Combustion
(Industrial) |
Production Processes |
Extraction &
Distribution of Fossil Fuels |
Solvent Use |
Road Transport |
Other Transport &
Machinery |
Waste Treatment &
Disposal |
Nature (Forests) |
TOTAL |
|
butane |
0.49 |
3.29 |
0.65 |
10.57 |
81.88 |
17.81 |
31.49 |
0.48 |
0.04 |
|
147 |
|
ethanol |
|
1.25 |
0.17 |
51.27 |
|
44.75 |
|
|
0.43 |
|
98 |
|
ethane |
1.29 |
4.26 |
0.22 |
3.93 |
49.49 |
|
6.16 |
0.59 |
5.27 |
|
71 |
|
2-methylbutane |
0.08 |
3.16 |
0.24 |
2.39 |
15.86 |
0.05 |
41.55 |
0.77 |
0.02 |
|
64 |
|
pentane |
0.19 |
1.89 |
0.69 |
6.38 |
29.57 |
0.44 |
21.00 |
0.30 |
0.03 |
|
60 |
|
propane |
0.48 |
2.47 |
0.29 |
5.77 |
37.23 |
3.49 |
2.55 |
0.39 |
5.27 |
|
58 |
|
toluene |
0.11 |
1.89 |
0.16 |
2.12 |
0.42 |
14.03 |
33.29 |
3.20 |
0.25 |
|
55 |
|
hexane |
0.14 |
0.28 |
0.09 |
6.12 |
15.18 |
2.77 |
19.61 |
0.21 |
0.16 |
|
45 |
|
ethylene |
0.24 |
2.78 |
0.52 |
9.03 |
0.11 |
|
25.25 |
4.11 |
|
|
42 |
|
2-methylpropane |
0.08 |
0.91 |
0.02 |
2.71 |
18.97 |
0.87 |
14.38 |
0.22 |
0.01 |
|
38 |
|
methanol |
|
|
0.03 |
1.51 |
|
30.06 |
|
|
0.11 |
|
32 |
|
m-xylene |
0.49 |
0.22 |
0.04 |
3.84 |
0.16 |
13.90 |
9.77 |
0.73 |
0.11 |
|
29 |
|
trichloroethene |
|
|
0.00 |
1.27 |
|
27.42 |
|
|
0.09 |
|
29 |
|
formaldehyde |
4.90 |
1.90 |
1.18 |
0.13 |
0.22 |
0.04 |
9.32 |
1.93 |
3.52 |
|
23 |
|
acetone |
0.07 |
0.03 |
0.07 |
1.83 |
|
19.65 |
1.31 |
0.13 |
0.00 |
|
23 |
|
heptane |
0.02 |
0.83 |
0.00 |
1.15 |
14.89 |
1.66 |
3.62 |
0.10 |
|
|
22 |
|
propylene |
0.30 |
1.30 |
0.06 |
3.53 |
0.05 |
|
12.65 |
1.53 |
|
|
19 |
|
ethylbenzene |
0.14 |
0.08 |
0.03 |
1.46 |
0.06 |
5.31 |
9.56 |
0.78 |
0.18 |
|
18 |
|
octane |
0.00 |
0.06 |
|
1.11 |
13.11 |
1.39 |
1.49 |
0.09 |
|
|
17 |
|
1,2,4-trimethylbenzene |
0.00 |
0.00 |
0.00 |
0.32 |
0.01 |
7.04 |
8.98 |
0.53 |
|
|
17 |
|
benzene |
0.19 |
3.15 |
0.82 |
1.65 |
0.67 |
|
8.40 |
1.53 |
0.06 |
|
16 |
|
o-xylene |
0.11 |
0.13 |
0.02 |
0.99 |
0.06 |
3.46 |
9.70 |
0.83 |
0.06 |
|
15 |
|
acetylene |
0.04 |
0.02 |
0.08 |
0.71 |
0.05 |
|
12.28 |
1.75 |
|
|
15 |
|
2-butanone |
|
|
0.00 |
0.06 |
|
14.04 |
0.35 |
0.02 |
0.02 |
|
14 |
|
butyl acetate |
|
|
|
0.05 |
|
13.39 |
|
|
0.03 |
|
13 |
|
p-xylene |
0.00 |
0.17 |
0.02 |
1.05 |
0.03 |
3.72 |
7.55 |
0.57 |
0.09 |
|
13 |
|
dichloromethane |
|
|
0.00 |
3.04 |
0.03 |
9.73 |
|
|
0.10 |
|
13 |
|
decane |
0.00 |
0.03 |
|
1.23 |
0.03 |
8.70 |
1.31 |
0.60 |
|
|
12 |
|
2-methylpropene |
0.00 |
0.16 |
0.00 |
0.08 |
0.42 |
|
9.72 |
1.13 |
0.01 |
|
12 |
|
ethyl acetate |
|
|
|
0.60 |
|
10.00 |
|
|
0.03 |
|
11 |
|
2-propanol |
|
0.01 |
|
0.76 |
|
9.43 |
|
|
0.02 |
|
10 |
|
2-butene |
0.01 |
0.64 |
0.00 |
0.38 |
1.29 |
|
5.72 |
0.22 |
0.03 |
|
8 |
|
4-methyl-2-pentanone |
|
|
|
0.12 |
|
7.18 |
|
|
|
|
7 |
|
1-butanol |
|
|
|
0.04 |
|
7.12 |
|
|
0.01 |
|
7 |
|
tetrachloroethene |
|
|
|
0.37 |
|
6.51 |
|
|
0.19 |
|
7 |
|
nonane |
0.00 |
0.05 |
|
0.82 |
0.09 |
5.26 |
0.34 |
0.15 |
|
|
7 |
|
2-pentene |
0.01 |
0.33 |
0.00 |
0.08 |
2.17 |
|
3.90 |
0.05 |
0.00 |
|
7 |
|
1,3,5-trimethylbenzene |
0.00 |
0.00 |
0.00 |
0.15 |
0.00 |
2.31 |
3.50 |
0.26 |
|
|
6 |
|
1,3-butadiene |
0.00 |
|
0.00 |
0.36 |
0.02 |
|
4.62 |
0.70 |
0.01 |
|
6 |
|
2-butoxyethanol |
|
|
|
0.00 |
|
5.36 |
|
|
|
|
5 |
|
undecane |
0.00 |
0.00 |
|
0.44 |
|
4.53 |
|
0.27 |
|
|
5 |
|
acetaldehyde |
0.00 |
0.00 |
0.00 |
0.17 |
|
|
4.03 |
0.83 |
|
|
5 |
|
2-methylpentane |
0.00 |
0.01 |
0.02 |
0.73 |
2.72 |
1.31 |
|
0.01 |
0.08 |
|
5 |
|
1,2,3-trimethylbenzene |
0.00 |
0.00 |
0.00 |
0.11 |
0.00 |
2.30 |
2.01 |
0.17 |
|
|
5 |
|
2-methylhexane |
|
|
|
0.10 |
0.16 |
0.91 |
3.16 |
0.21 |
|
|
5 |
|
methyl acetate |
|
|
|
4.20 |
|
0.00 |
|
|
|
|
4 |
|
methylethylbenzene |
|
|
|
|
|
4.07 |
|
|
|
|
4 |
|
1-butene |
0.02 |
0.30 |
0.05 |
0.43 |
0.39 |
|
2.64 |
0.17 |
0.01 |
|
4 |
|
1-propanol |
|
|
|
|
|
3.92 |
|
|
|
|
4 |
|
1-methoxy-2-propanol |
|
|
|
|
|
3.84 |
|
|
|
|
4 |
|
Total |
9.42 |
31.63 |
5.49 |
135.17 |
285.34 |
317.79 |
331.19 |
25.53 |
16.33 |
0.00 |
1158 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
unspeciated |
0.02 |
1.83 |
2.60 |
38.48 |
2.38 |
10.68 |
1.64 |
0.41 |
0.18 |
|
58 |
|
isoprene + BVOC 1 |
|
|
|
|
|
|
|
|
|
178.0 |
178 |
|
other grouped species |
0.01 |
0.72 |
0.01 |
4.57 |
10.77 |
7.84 |
53.79 |
33.28 |
1.95 |
|
113 |
|
other VOC |
0.25 |
1.70 |
0.19 |
28.98 |
4.03 |
108.21 |
21.42 |
3.53 |
2.38 |
|
171 |
|
Total VOC |
10 |
36 |
8 |
207 |
303 |
445 |
408 |
63 |
21 |
178 |
1678 |
1”BVOC” are biogenic VOCs. An entry of "0.00" represents a value of <0.005
ktonnes (i.e. <5 tonnes)
Photochemical Ozone Creation Potential
The speciation given in Table 5.7 (and Appendix 5) is a useful reference for finding the emission of a particular NMVOC compound. However, this does not reflect the fact that different NMVOC compounds are more or less efficient at generating ozone through photochemical reactions. To resolve this, the concept of a photochemical ozone creation potential (POCP) was created. This POCP identifies, on a relative basis, the ozone creation potential for each NMVOC compound through modelling studies. The creation potentials are then normalised by defining ethene as a creation potential of 1.
It is therefore possible to determine which NMVOCs are the more important for the photochemical formation of ozone in the atmosphere. This is achieved by scaling the emissions of each NMVOC by the corresponding POCP to determine a weighted total.
Table 5.8 POCP Weighted NMVOC Emissions
|
|
POCP |
Code |
Stationary Combustion |
Production Processes |
Extraction and Distribution of Fossil
Fuels |
Solvent Use |
Road Transport |
Other Transport & Machinery |
Waste Treatment & Disposal |
Nature (Forests) |
TOTAL (Mass Emission) |
TOTAL (POCP Weighted) |
TOTAL (POCP Weighted %) |
|
butane |
35.2 |
a |
1.56 |
3.72 |
28.82 |
6.27 |
11.09 |
0.17 |
0.01 |
|
147 |
52 |
5.8% |
|
ethylene |
100.0 |
a |
3.54 |
9.03 |
0.11 |
|
25.25 |
4.11 |
|
|
42 |
42 |
4.7% |
|
ethanol |
39.9 |
a |
0.57 |
20.46 |
|
17.86 |
|
|
0.17 |
|
98 |
39 |
4.4% |
|
toluene |
63.7 |
a |
1.38 |
1.35 |
0.27 |
8.94 |
21.21 |
2.04 |
0.16 |
|
55 |
35 |
4.0% |
|
m-xylene |
110.8 |
a |
0.84 |
4.26 |
0.18 |
15.41 |
10.83 |
0.81 |
0.13 |
|
29 |
32 |
3.7% |
|
2-methylbutane |
40.5 |
a |
1.41 |
0.97 |
6.42 |
0.02 |
16.83 |
0.31 |
0.01 |
|
64 |
26 |
2.9% |
|
pentane |
39.5 |
a |
1.09 |
2.52 |
11.68 |
0.17 |
8.30 |
0.12 |
0.01 |
|
60 |
24 |
2.7% |
|
propylene |
112.3 |
a |
1.87 |
3.97 |
0.06 |
|
14.21 |
1.72 |
|
|
19 |
22 |
2.5% |
|
1,2,4-trimethylbenzene |
127.8 |
a |
0.00 |
0.41 |
0.01 |
9.00 |
11.47 |
0.68 |
|
|
17 |
22 |
2.4% |
|
hexane |
48.2 |
a |
0.25 |
2.95 |
7.32 |
1.33 |
9.45 |
0.10 |
0.08 |
|
45 |
21 |
2.4% |
|
o-xylene |
105.3 |
a |
0.27 |
1.04 |
0.06 |
3.64 |
10.22 |
0.87 |
0.07 |
|
15 |
16 |
1.8% |
|
p-xylene |
101.0 |
a |
0.20 |
1.06 |
0.03 |
3.76 |
7.63 |
0.57 |
0.09 |
|
13 |
13 |
1.5% |
|
ethylbenzene |
73.0 |
a |
0.18 |
1.06 |
0.05 |
3.88 |
6.98 |
0.57 |
0.13 |
|
18 |
13 |
1.4% |
|
formaldehyde |
51.9 |
a |
4.14 |
0.07 |
0.11 |
0.02 |
4.84 |
1.00 |
1.82 |
|
23 |
12 |
1.4% |
|
2-methylpropane |
30.7 |
a |
0.31 |
0.83 |
5.82 |
0.27 |
4.41 |
0.07 |
0.00 |
|
38 |
12 |
1.3% |
|
heptane |
49.4 |
a |
0.42 |
0.57 |
7.35 |
0.82 |
1.79 |
0.05 |
|
|
22 |
11 |
1.2% |
|
propane |
17.6 |
a |
0.57 |
1.01 |
6.55 |
0.62 |
0.45 |
0.07 |
0.93 |
|
58 |
10 |
1.1% |
|
2-butene |
113.9 |
a |
0.74 |
0.44 |
1.47 |
|
6.51 |
0.25 |
0.03 |
|
8 |
9 |
1.1% |
|
trichloroethene |
32.5 |
a |
0.00 |
0.41 |
|
8.91 |
|
|
0.03 |
|
29 |
9 |
1.1% |
|
ethane |
12.3 |
a |
0.71 |
0.48 |
6.09 |
|
0.76 |
0.07 |
0.65 |
|
71 |
9 |
1.0% |
|
1,3,5-trimethylbenzene |
138.1 |
a |
0.00 |
0.20 |
0.00 |
3.20 |
4.83 |
0.36 |
|
|
6 |
9 |
1.0% |
|
octane |
45.3 |
a |
0.03 |
0.50 |
5.94 |
0.63 |
0.67 |
0.04 |
|
|
17 |
8 |
0.9% |
|
2-pentene |
111.9 |
a |
0.39 |
0.09 |
2.43 |
|
4.36 |
0.05 |
0.00 |
|
7 |
7 |
0.8% |
|
2-methylpropene |
62.7 |
a |
0.10 |
0.05 |
0.26 |
|
6.10 |
0.71 |
0.00 |
|
12 |
7 |
0.8% |
|
1,2,3-trimethylbenzene |
126.7 |
a |
0.00 |
0.14 |
0.00 |
2.91 |
2.54 |
0.22 |
|
|
5 |
6 |
0.7% |
|
2-butanone |
37.3 |
a |
0.00 |
0.02 |
|
5.24 |
0.13 |
0.01 |
0.01 |
|
14 |
5 |
0.6% |
|
1,3-butadiene |
85.1 |
a |
0.00 |
0.31 |
0.02 |
|
3.93 |
0.60 |
0.01 |
|
6 |
5 |
0.5% |
|
decane |
38.4 |
a |
0.01 |
0.47 |
0.01 |
3.34 |
0.50 |
0.23 |
|
|
12 |
5 |
0.5% |
|
1-butanol |
62.0 |
a |
|
0.02 |
|
4.41 |
|
|
0.01 |
|
7 |
4 |
0.5% |
|
methanol |
14.0 |
a |
0.00 |
0.21 |
|
4.21 |
|
|
0.02 |
|
32 |
4 |
0.5% |
|
1-butene |
107.9 |
a |
0.40 |
0.47 |
0.42 |
|
2.85 |
0.18 |
0.01 |
|
4 |
4 |
0.5% |
|
methylethylbenzene |
94.1 |
c |
|
|
|
3.83 |
|
|
|
|
4 |
4 |
0.4% |
|
butyl acetate |
26.9 |
a |
|
0.01 |
|
3.60 |
|
|
0.01 |
|
13 |
4 |
0.4% |
|
benzene |
21.8 |
a |
0.91 |
0.36 |
0.15 |
|
1.83 |
0.33 |
0.01 |
|
16 |
4 |
0.4% |
|
4-methyl-2-pentanone |
49.0 |
a |
|
0.06 |
|
3.52 |
|
|
|
|
7 |
4 |
0.4% |
|
acetaldehyde |
64.1 |
a |
0.00 |
0.11 |
|
|
2.58 |
0.53 |
|
|
5 |
3 |
0.4% |
|
ethyldimethylbenzene |
132.0 |
c |
|
|
|
3.12 |
|
|
|
|
2 |
3 |
0.4% |
|
1-pentene |
97.7 |
a |
0.14 |
0.14 |
0.44 |
|
2.32 |
0.06 |
0.00 |
|
3 |
3 |
0.3% |
|
nonane |
41.4 |
a |
0.02 |
0.34 |
0.04 |
2.18 |
0.14 |
0.06 |
|
|
7 |
3 |
0.3% |
|
2-butoxyethanol |
48.3 |
a |
|
0.00 |
|
2.59 |
|
|
|
|
5 |
3 |
0.3% |
|
dipentene |
74.54 |
b |
|
|
|
2.45 |
|
|
|
|
3 |
2 |
0.3% |
|
1-propanol |
56.1 |
a |
|
|
|
2.20 |
|
|
0.03 |
|
4 |
2 |
0.3% |
|
ethyl acetate |
20.9 |
a |
|
0.13 |
|
2.09 |
|
|
0.01 |
|
11 |
2 |
0.3% |
|
acetone |
9.4 |
a |
0.02 |
0.17 |
|
1.85 |
0.12 |
0.01 |
0.00 |
|
23 |
2 |
0.2% |
|
2-methylpentane |
42.0 |
a |
0.01 |
0.31 |
1.14 |
0.55 |
|
0.01 |
0.03 |
|
5 |
2 |
0.2% |
|
undecane |
38.4 |
a |
0.00 |
0.17 |
|
1.74 |
|
0.10 |
|
|
5 |
2 |
0.2% |
|
2-propanol |
18.8 |
a |
0.00 |
0.14 |
|
1.77 |
|
|
0.00 |
|
10 |
2 |
0.2% |
|
2-methylhexane |
41.1 |
a |
|
0.04 |
0.07 |
0.37 |
1.30 |
0.08 |
|
|
5 |
2 |
0.2% |
|
1,3-hexadiene |
103.7 |
b |
|
|
|
|
1.64 |
|
|
|
2 |
2 |
0.2% |
|
3-methylpentane |
47.9 |
a |
0.01 |
0.26 |
0.70 |
0.49 |
|
|
0.03 |
|
3 |
1 |
0.2% |
|
Total Top 50 (POCP) |
|
|
22.12 |
61.33 |
94.01 |
137.20 |
208.05 |
17.16 |
4.51 |
0.00 |
1128 |
544 |
61% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Speciated VOCs |
|
|
1.34 |
10.01 |
1.16 |
50.64 |
8.19 |
1.68 |
0.85 |
0.00 |
200 |
74 |
8% |
|
Isoprene and BVOCs 1 |
90.0 |
c |
|
|
|
|
|
|
|
160.2 |
178 |
160 |
18% |
|
Other grouped species |
|
|
0.63 |
2.31 |
4.87 |
3.99 |
48.68 |
18.29 |
1.07 |
|
113 |
80 |
9% |
|
Unspeciated VOCs |
51.3 |
c |
2.29 |
19.74 |
1.22 |
5.48 |
0.84 |
0.21 |
0.09 |
|
58 |
30 |
3% |
|
Total- All VOC |
|
|
26 |
93 |
101 |
197 |
266 |
37 |
7 |
160 |
1678 |
888 |
100% |
1”BVOC” are biogenic VOCs. An entry of "0" represents a value
of <0.005 ktonnes (i.e. < 5 tonnes)
Temporal Disaggregation of NMVOC Emission Estimates
The emission of NMVOC plays a key role the formation of ground level ozone. The representation of the emissions therefore has an important influence on the results of emission-driven ozone modelling studies. In addition to the overall magnitude and speciation of the emissions, it is also important to define their temporal variation.
Broadly speaking, the emissions can vary on three timescales, namely (i) with season, (ii) with day of week, and (iii) with hour of day. Clearly, a correct description of the seasonal dependence is important, because the photochemical conditions required for ozone formation occur during the summer months (April – September). The hour of day dependence is also important, particularly for very reactive NMVOC which are rapidly oxidised during daylight hours. The variation of emissions with day of week, in conjunction with the multi-day timescale for ozone formation and transport to occur, is believed to provide an explanation for the observed prevalence of photochemical ozone episodes on particular days of the week (Jenkin et al., 2000).
Variations in emissions of NMVOC have been estimated over the following timescales:
· Diurnal: the portion of daily emissions which occur during each hour of a typical 24 hour period have been estimated.
· Weekly: the portion of weekly emissions which occur on each day of a typical week have been estimated.
· Annual: the portion of annual emissions which occur during each month of a typical year have been estimated.
A single representative profile has therefore been defined for the diurnal, weekly and annual variations for each NMVOC source category. Currently, no attempt has been made to identify differences in the diurnal pattern of emissions on different days of the week or at different times of year. Similarly, no attempt has been made to distinguish differences in the weekly pattern of emissions at different times of year. Such differences could well exist: for example, it is conceivable that emissions from decorative paint use and lawn mowers could exhibit a different pattern on a weekend compared with a week-day. In the former case, the emission might be expected to occur throughout the day whereas, in the latter case, emissions might be expected to peak during the early evening, after many people return home from work. Clearly, a fully rigorous temporal profile should therefore define the portion of emissions occurring in each hour of a given year, with the precise profile also changing from one year to the next. However, such a methodology would be impractical, and the resultant information would be difficult to use in modelling applications. The present approach is therefore designed to provide a practical method of defining the temporal variations in emissions.
Two approaches have been used to define the temporal
profiles – the use of ‘real’ data or the use of ‘default’ profiles. In the first case, we have used data such as
fuel consumption, electricity generated, or traffic volumes, which can be
related to emissions, and which are themselves temporally resolved. In the second case, we have assumed that
emissions follow one of a small set of default profiles. This second approach is used for most
emission sources since no ‘real’ data are available. The default profiles have been applied, based on our knowledge of
the emitting processes. Real data were used to define the typical
temporal variations for a series of categories, namely road transport, natural
emissions (forests), paint sectors and power generation. Further details of both the default profiles
and real data used for temporal disaggregation of VOC emissions are given in
Goodwin et al, 2001.
Total NMVOC temporal profiles
The applied temporal
codes allow emissions estimates to be made for a given hour for either total
NMVOC, or for specific sectors or sectoral combinations. Some examples of data
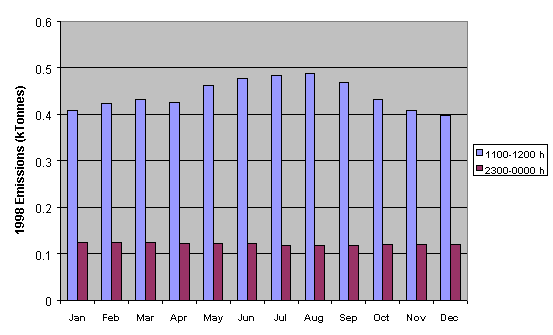
for total NMVOC emissions are presented in Figures 5.12-5.14. Figure 5.12 shows a comparison of the
emissions of total NMVOC on a typical Friday in each month of the year, both
for the hour ending at midday and the hour ending at midnight. Whereas the
night-time emissions estimates show little variation with month, the daytime
emissions maximise in the summer, owing partially to increased emissions from
road transport.
Figure 5.12 Emissions of
NMVOC for the Hours Ending at Midday and Midnight on a Typical Friday in Each
Month of the Year.
Figure
5.13: Emissions of NMVOC for the Hours Ending at Midday and Midnight for Each
Day of the Week in July.
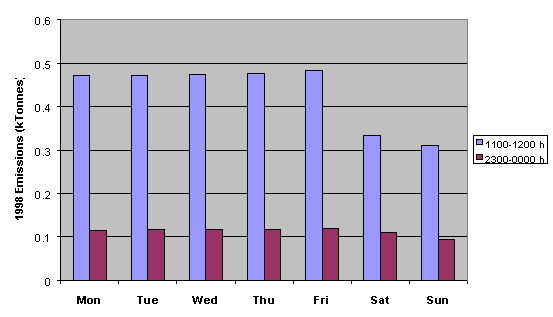
Figure 5.14 Emissions of
NMVOC for Each Hour of the Day for a Typical Friday in January and July.
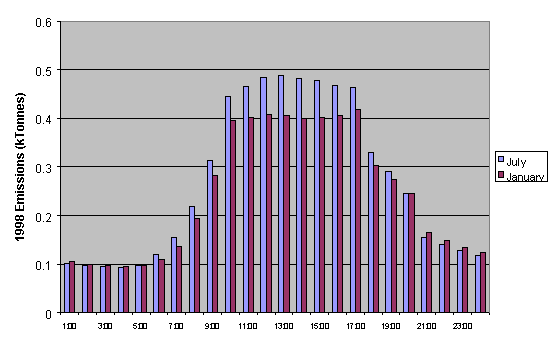
Figure 5.13 demonstrates that NMVOC emissions are substantially greater on weekdays than they are on weekends. This information is currently being applied in ozone modelling studies to investigate the possible link between the day-of-week variation in ozone precursor emissions and the observed prevalence of episodes of health-related ozone threshold concentration exceedances on particular days of the week.
A typical diurnal dependence of total NMVOC emissions is
presented in Figure 5.14 for midsummer and midwinter. The calculated profiles
are reasonably similar, which partially reflects that only a single
representative profile is assigned to each source with no allowance made for
possible variations with time of year. Also, the diurnal dependence of the
major contributions from road transport and many solvent subcategories are
strongly correlated with the working day, such that the similarity in the
summer and winter profiles would seem reasonable.
Ammonia Emission estimates
Emissions from the agricultural sector are taken directly from the agricultural NH3 inventory compiled for LMID (a part of Defra) each year by a consortium of organisations. There is on-going work to improve the NH3 emission estimates from both agricultural and non-agricultural sources.
Table 5.11 UK Emissions of Ammonia (ktonnes)
|
|
1970 |
1980 |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2000% |
|
BY UN/ECE CATEGORY1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comb. in Energy
Prod. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0% |
|
Other Comb. & Trans. |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0% |
|
Comb. in
Comm/Inst/Res |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Residential Plant |
|
|
5 |
6 |
5 |
6 |
5 |
4 |
4 |
4 |
3 |
4 |
3 |
1% |
|
Comm/Pub/Agri Comb |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0% |
|
Combustion in
Industry |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iron & Steel Comb. |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0% |
|
Other Ind. Comb. |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0% |
|
Production
Processes |
|
|
8 |
8 |
8 |
8 |
8 |
7 |
8 |
6 |
9 |
5 |
4 |
1% |
|
Solvent Use |
|
|
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
0% |
|
Road Transport |
|
|
1 |
1 |
2 |
4 |
6 |
9 |
12 |
12 |
12 |
12 |
12 |
4% |
|
Off road sources |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0% |
|
Waste |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Landfill |
|
|
8 |
8 |
8 |
7 |
7 |
7 |
6 |
6 |
6 |
5 |
5 |
2% |
|
Non Landfill Waste |
|
|
6 |
6 |
5 |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
2% |
|
Agriculture |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Animal Wastes |
|
|
257 |
255 |
254 |
256 |
258 |
252 |
257 |
259 |
253 |
250 |
237 |
74% |
|
Non Livestock Agricul. |
|
|
54 |
58 |
44 |
39 |
37 |
32 |
26 |
30 |
29 |
33 |
28 |
9% |
|
Nature |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Wild Animal Wastes |
|
|
6 |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
2% |
|
Humans |
|
|
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
1% |
|
Other Animal Wastes |
|
|
16 |
16 |
16 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
15 |
5% |
|
By FUEL TYPE |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Solid |
|
|
5 |
6 |
5 |
6 |
5 |
4 |
4 |
4 |
3 |
4 |
3 |
1% |
|
Petroleum |
|
|
1 |
1 |
2 |
4 |
6 |
9 |
12 |
12 |
12 |
12 |
12 |
4% |
|
Gas |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0% |
|
Non-Fuel |
|
|
357 |
358 |
342 |
339 |
338 |
327 |
327 |
331 |
326 |
322 |
304 |
95% |
|
TOTAL |
|
|
363 |
365 |
349 |
348 |
349 |
339 |
342 |
347 |
341 |
338 |
319 |
100% |
1 See Annex 1 for definition of UN/ECE Categories
Ammonia emissions are dominated by agricultural sources with emissions from livestock and their wastes comprising 74% of the total emission. These emissions derive mainly from the decomposition of urea in animal wastes and uric acid in poultry wastes. Emissions depend on animal species, age, weight, diet, housing systems, waste management and storage techniques. Hence emissions are affected by a large number of factors which make the interpretation of experimental data difficult and emission estimates uncertain (DOE, 1994). The other agricultural sources included are emissions from fertiliser use, crops and decomposition of agricultural vegetation. These are particularly uncertain owing to the complexity of the processes involved.
Sutton et al (2000a, 2000b) give estimates of a number of non-agricultural emission estimates, some of which have been incorporated here. The non agricultural sources comprise a number of diverse sources and equal 17% of the total. However, emission estimates for these sources are very uncertain due to a lack of data. Emissions of ammonia from road transport although relatively small are increasing as a result of the increasing number of three way catalysts in the vehicle fleet. Detailed consideration has been given to the current emissions and potential future emissions from non-agricultural sources in Handley et al (2001).
Figure 5.18 Ammonia Emissions Profile
Figure 5.19, Spatially Disaggregated UK Emissions of NH3
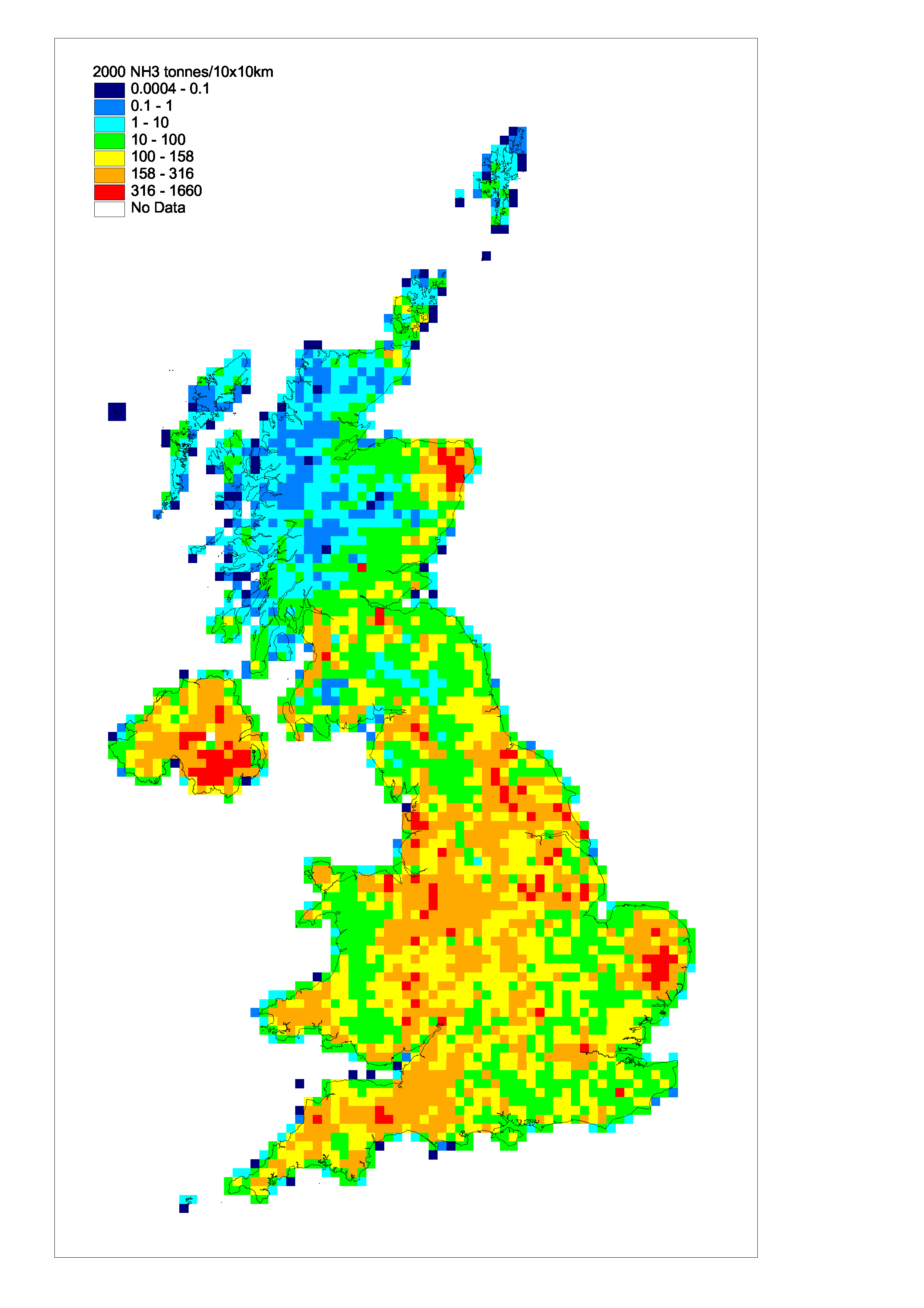
Hydrogen FlUoride emission estimates
Hydrogen fluoride (HF) is an acidic gas released to air from combustion of fuels which contain trace amounts of fluoride. Some industrial processes use HF as a an acidic reagent (or produce HF), giving rise to emissions. HF is chemically very similar to HCl.
Hydrogen fluoride emissions for the UK are presented here for the first time. As expected, the emissions of HF displays a similar source pattern to HCl (see Section 5.4). However, the emissions of HF from the power generation sector do not account for such a high percentage of the total (see Table 5.12). The reduction of the emissions from this sector with time is an indication of the increased use of emissions abatement equipment. Emissions of HF from the residential sector are noted to have decreased with time. This is due to the decreasing use of coal in domestic heating. These trends with time are highlighted in Figure 5.20.
The increase in HF emission between 1999 and 2000 is caused by the increased coal consumption in electricity generation. Interestingly this trend is not noted for HCl (which exhibits a decrease from 1999 to 2000). This is because the HCl emission per unit of coal consumed decreased between 1999 and 2000, whereas that for HF remained reasonably constant.
Table 5.12 UK Emissions of Hydrogen Fluoride by UN/ECE Source Category and Fuel (kt)
|
|
1970 |
1980 |
1990 |
1991 |
1992 |
1993 |
1994 |
1995 |
1996 |
1997 |
1998 |
1999 |
2000 |
2000% |
|
BY UN/ECE CATEGORY1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comb. in Energy
Prod. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Public Power |
6.1 |
7.1 |
6.6 |
6.5 |
6.1 |
5.1 |
4.6 |
3.5 |
2.9 |
2.0 |
2.1 |
1.8 |
2.0 |
52% |
|
Other Comb. & Trans. |
2.8 |
1.1 |
1.0 |
0.9 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
0.8 |
20% |
|
Comb. in Comm/Inst/Res |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Residential Plant |
1.8 |
0.8 |
0.4 |
0.4 |
0.4 |
0.4 |
0.4 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
4% |
|
Comm/Pub/Agri Comb. |
0.4 |
0.2 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.0 |
0.1 |
0.1 |
0.0 |
0.0 |
0.0 |
1% |
|
Combustion in
Industry |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iron & Steel Comb. |
0.1 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0.0 |
0% |
|
Other Ind. Comb. |
2.4 |
1.2 |
0.9 |
0.9 |
0.9 |
0.8 |
0.8 |
0.8 |
0.7 |
0.7 |
0.6 |
0.5 |
0.4 |
12% |
|
Production
Processes |
0.5 |
1.8 |
1.3 |
1.4 |
1.1 |
1.1 |
1.1 |
1.1 |
1.1 |
1.1 |
1.1 |
0.4 |
0.4 |
11% |
|
TOTAL |
14.12 |
12.2 |
10.3 |
10.2 |
9.4 |
8.3 |
7.6 |
6.5 |
5.8 |
4.9 |
4.9 |
3.7 |
3.8 |
100% |
1 See Annex 1 for definition of UN/ECE Categories
Figure 5.20 Hydrogen Fluoride Emissions Profile
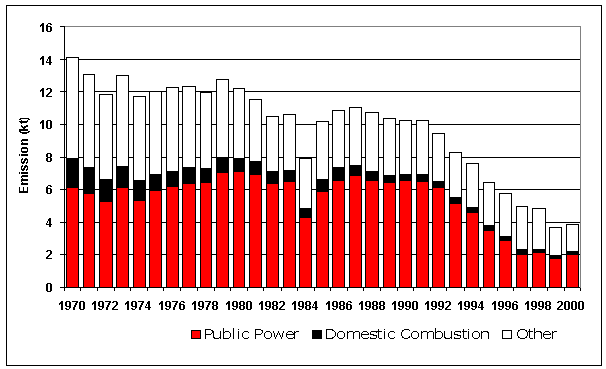
ACCURACY of emission estimates of Acidifying Gases and Tropospheric ozone precursors
Quantitative estimates of the uncertainties in emission inventories have been based on calculations made using a direct simulation technique, which corresponds to the IPCC Tier 2 approach recommended for greenhouse gases and also the methodology proposed in draft guidance produced by the UN ECE Taskforce on Emission Inventories. This work is described in detail by Passant (2002). Uncertainty estimates are shown in Table 5.13.
Table 5.13 Uncertainty of the Emission Inventories
|
Pollutant |
Estimated
Uncertainty % |
|
Sulphur Dioxide |
+/- 3% |
|
Oxides of Nitrogen |
+/- 7% |
|
Non-Methane Volatile Organic Compounds |
+/- 10% |
|
Ammonia |
+/- 20% |
|
Hydrogen Chloride |
+/- 20% |
|
Hydrogen Fluoride |
+/- 20%a |
a - assumed to be same as for hydrogen chloride (see text for discussion)
Sulphur Dioxide
Sulphur dioxide emissions can be estimated with most confidence as they depend largely on the level of sulphur in fuels. Hence the inventory, being based upon comprehensive analysis of coals and fuel oils consumed by power stations and the agriculture, industry and domestic sectors, contains accurate emission estimates for the most important sources.
Oxides of Nitrogen
NOx emission estimates are less accurate than SO2 because they are based on measured emission factors and emission rates can vary widely with combustion conditions. In the case of road transport emissions, while the inventory methodology takes into account variations in the amount of NOx emitted as a function of speed and vehicle type, significant variations in measured emission factors have been found even when keeping these parameters constant. Emission factors given in the literature for stationary combustion sources also show large variation.
From the above, one might expect the NOx inventory to be very uncertain, however the overall uncertainty is in fact lower than any pollutant other than SO2. This is probably largely as a result of two factors. Firstly, while emission factors are uncertain, activity data used in the NOx inventory is very much less uncertain. This contrasts with inventories for pollutants such as volatile organic compounds, PM10, metals, and persistent organic pollutants, where some of the activity data are very uncertain. Secondly, the NOx inventory is made up of a large number of emission sources with many of similar size and with none dominating (the most important source contributes just 18% of emissions, and a further 42 sources must be included to cover 90% of the emission). This leads to a large potential for error compensation, where an underestimate in emissions in one sector is very likely to be compensated by an overestimate in emissions in another sector. The other extreme is shown by the inventories for PCP, HCH and HCB (see Section 6.2.4) where one or two sources dominate and the inventories are highly uncertain.
Non-Methane Volatile
Organic Compounds (NMVOC)
The NMVOC inventory is less uncertain than those for SO2 and NOx. This is due in part to the difficulty in obtaining good emission factors or emission estimates for some sectors (e.g. fugitive sources of VOC emissions from industrial processes, and natural sources) and partly due to the absence of good activity data for some sources.
As with NOx, there is a high potential for error compensation, and this is responsible for the relatively low level of uncertainty compared with most other pollutants in the NAEI.
Ammonia
Ammonia emission estimates are more uncertain than those for SO2, NOx and NMVOC due largely to the nature of the major agricultural sources. Emissions depend on animal species, age, weight, diet, housing systems, waste management and storage techniques. Hence emissions are affected by a large number of factors which make the interpretation of experimental data difficult and emission estimates uncertain (DOE, 1994). Emission estimates for non-agricultural sources such as wild animals are also highly uncertain. Unlike the case of NOx and NMVOC, a few sources dominate the inventory and there is limited potential for error compensation.
Hydrogen Chloride
The hydrogen chloride inventory is equally uncertain as the ammonia inventory. As with ammonia, a few sources dominate the inventory and the levels of uncertainty in these sources is generally quite high.
Hydrogen Fluoride
Uncertainty analysis has not been performed on the hydrogen fluoride inventory as this is not a core part of the NAEI. However, the sources of hydrogen fluoride are very similar to those for hydrogen chloride and the level of uncertainty in emission factors might also be expected to be similar. As a result it seems reasonable to assume the same level of overall uncertainty as for hydrogen chloride.